Robert Giugliano, MD, on the Use of NOACs Among Patients With Obesity
New data1 are shedding light on the use of nonvitamin K antagonist oral anticoagulants (NOACs) among patients with obesity.
While 4 NOACs are currently approved for reducing the risk of stroke and systemic embolism among patients with atrial fibrillation (AF), data on the drugs’ use in patients with obesity are limited.
Now, new research shows preserved efficacy with NOACs vs warfarin—with similar risk of major bleeding—in patients with obesity. According to the researchers, the efficacy and safety of apixaban or edoxaban appear to be similar to warfarin in patients with a body mass index (BMI) of 40 to 50 kg/m2.
The researchers suggest that the new data—obtained through analyses of 4 trials that compared NOAC and warfarin—should be considered in updated guidelines.
Robert P. Giugliano, MD, a cardiovascular medicine specialist at Brigham and Women’s Hospital and an associate professor of medicine at Harvard Medical School, was one of the researchers. Here, he answers Cardiology Consultant’s questions about the current limitations of AF management guidelines, how he envisions this data impacting those guidelines, and how you can implement the findings into your practice.
CARDIOLOGY CONSULTANT: You wrote that the current guidelines have limited and conflicting recommendations regarding the use of NOACs among patients with obesity. Can you expand on this?
Robert Giugliano: On page 6 of our paper, in the section entitled “Guidelines/Recommendations,” we note the following:
- The 2019 American Heart Association/American College of Cardiology/Heart Rhythm Society guideline2 recommends measuring NOAC levels if the patient has a BMI of more than 40 kg/m2 or weighs more than 120 kg.
- The 2018 European Heart Rhythm Association recommends3 vitamin K antagonists (VKAs) instead of NOACs in patients with severe obesity (BMI >40 kg/m2).
- The Working Group on Thrombosis suggests4 switching to a VKA rather than adjusting the NOAC dose if the trough levels are subtherapeutic. It is also noted that it is OK to use NOACs if the trough levels are in the therapeutic range.
- The 2018 guidance from the American College of Clinical Pharmacy provides no specific guidelines.
- The 2016 European Society of Cardiology guideline5 for AF management offers no specific recommendations.
As you can see, the recommendations vary with regard to which anticoagulants to consider and whether measuring trough levels of a NOAC should be done; and 2 of the 5 offer no advice.
CARDIO CON: What role do you envision your new data playing in the development of updated guidelines?
RG: We believe our data that summarize the efficacy and safety data with NOACs, as well as the data we have provided on NOAC drug levels, could lead to new recommendations for their use in select patients with obesity. Specifically, we proposed the following based on the data.
If newly starting an anticoagulant in a patient with obesity:
- If the BMI is less than 40 kg/m2, then any of the 4 NOACs at the approved dose could be used.
- If the BMI is 40 to 49 kg/m2, then we recommend apixaban or edoxaban.
- If the BMI is 50 kg/m2 or higher, then we recommend either using a VKA or measuring plasma trough concentration of a NOAC to ensure the level is in the therapeutic range.
In addition, because there are limited data with dabigatran and rivaroxaban in patients with a BMI of 40 to 49 kg/m2, if a patient in this weight group is already being treated with dabigatran or rivaroxaban, then we suggest 1 of the 3 following steps:
- Measure plasma concentration of dabigatran or rivaroxaban. If the level is therapeutic, then it is OK to continue. If it is subtherapeutic, then switch to a different agent (VKA, apixaban, edoxaban); or
- Switch to VKA; or
- Switch to apixaban or edoxaban.
Note: If a provider cannot or does not want to measure drug levels (option 1), then he or she could simply switch to VKA, apixaban, or edoxaban. We would not advise continuing dabigatran or rivaroxaban in absence of drug levels in patients with BMIs of more than 40 kg/m2.
CARDIO CON: How do you suggest that your peers implement these findings into their current practice?
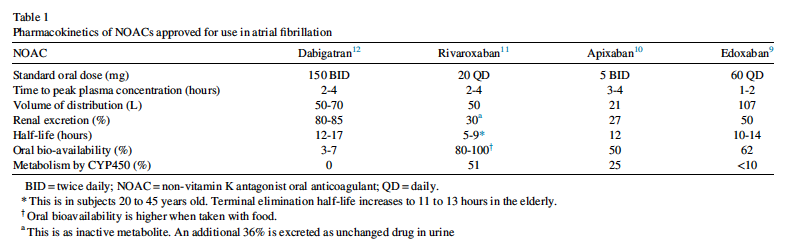
RG: I suggest that they review the data and carefully select anticoagulants in patients with BMI of more than 40 kg/m2 based on the available data. One common theme across the many papers from the NOAC vs warfarin trials is that one should not assume that the 4 NOACs are interchangeable. As shown in our Table 1, there are important differences in pharmacokinetic/pharmacodynamic properties, drug-drug interactions, and in the randomized controlled trials comparing NOAC vs warfarin, so it is important for prescribers to learn about the differences between the agents.
CARDIO CON: What should cardiologists consider when deciding between prescribing a NOAC or warfarin at therapy initiation for a patient with obesity and AF? What should they consider when switching such a patient from a NOAC to warfarin?
RG: In addition to the data in patients with obesity and our recommendations above, clinicians should also consider several other specific features of the patient, among the most important of which are:
- Risk of bleeding (edoxaban and apixaban significantly reduced major bleeding compared with warfarin; rivaroxaban and dabigatran did not)
- Concomitant therapies and the potential for drug-drug interactions (eg, rivaroxaban and apixaban are metabolized by the cytochrome P450 system; dabigatran and edoxban have no or minimal metabolism by this enzymatic system)
- Patient preferences regarding the need for frequent blood testing with VKA, how important food-drug interactions are (need to have a steady diet with VKA; rivaroxaban must be taken with food), once daily (apixaban, edoxaban, VKA) vs twice daily (dabigatran, rivaroxaban)
- Costs (VKAs are generic)
CARDIO CON: What are some challenges you anticipate clinicians, patients, or guideline panels facing in relation to the adoption of NOAC use among patients with AF who are obese?
RG: For clinicians: time pressure to discuss with patients the nuances between the options, closing the knowledge gap about differences between anticoagulants and what the data specifically show with NOACs, and the lack of availability of assays to measure drug levels.
For patients: comfort with existing therapy (“the devil I know”) vs switching, misperceptions about the inability to reverse the anticoagulant effects of NOACs, and the increased cost of NOACs vs generic VKA.
For guidelines: limited data comparing NOACs and very little data in patients with BMI greater than 50 kg/m2, tendency for some guidelines to avoid preference of one drug over another within the same drug class (especially in the United States), and balancing what is ideal (eg, measuring drug levels) with what is practical (almost no center can do this outside of specialized research sites).
References:
- Wang SY, Giugliano RP. Non-vitamin K antagonist oral anticoagulant for atrial fibrillation in obese patients. Am J Cardiol. Published online April 23, 2020. doi:10.1016/j.amjcard.2020.04.016
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2). doi:10.1161/CIR.0000000000000665
- Steffel J, Verhamme P, Potpara TS, et al; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330-1393. doi:10.1093/eurheartj/ehy136
- Rocca B, Fox KAA, Ajjan RA, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J. 2018;39(19):1672-1686. doi:10.1093/eurheartj/ehy066
- Kirchhof P, Benussi S, Kotecha D, et al; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. https://doi.org/10.1093/eurheartj/ehw210


