Sulfonylurea-Induced Hypoglycemia: The Case Against Glyburide
Since it came onto the market in 1983, glyburide has been one of the most popular sulfonylureas. The American Diabetes Association (ADA) recommends the use of these agents as part of a stepwise approach to treating type 2 diabetes mellitus. According to the ADA algorithm, all patients should start with therapeutic lifestyle modifications and metformin—if no contraindications are present. Next, depending on the hemoglobin A1c (HbA1c) level, patients should receive either a sulfonylurea or insulin.1
Sulfonylureas were among the first oral antihyperglycemic agents developed, and they are still commonly used today because they are inexpensive and well tolerated.2 They produce an antihyperglycemic effect by stimulating insulin secretion from the pancreas; it therefore follows that hypoglycemia is the chief adverse effect associated with their use.3
Glyburide, a second-generation sulfonylurea, is associated with hypoglycemia more often than most other sulfonylureas.4 Currently, glyburide is not recommended for patients with renal impairment (creatinine clearance of less than 50 mL/min) or severe hepatic impairment because of the risk of hypoglycemia. It should be used with caution in elderly patients because they are also at increased risk for hypoglycemia.
Here we will examine the evidence to address the question of whether glyburide should still be prescribed when other equally inexpensive second-generation sulfonylureas that pose less risk of hypoglycemia, such as glipizide and glimepiride, are available.
HYPOGLYCEMIA WITH SULFONYLUREAS
Studies have been conducted to determine which sulfonylureas are the safest and most effective. Because hypoglycemia is the chief adverse effect of sulfonylurea use, many studies have looked specifically at this phenomenon. Table 1 summarizes the results of some of these trials.
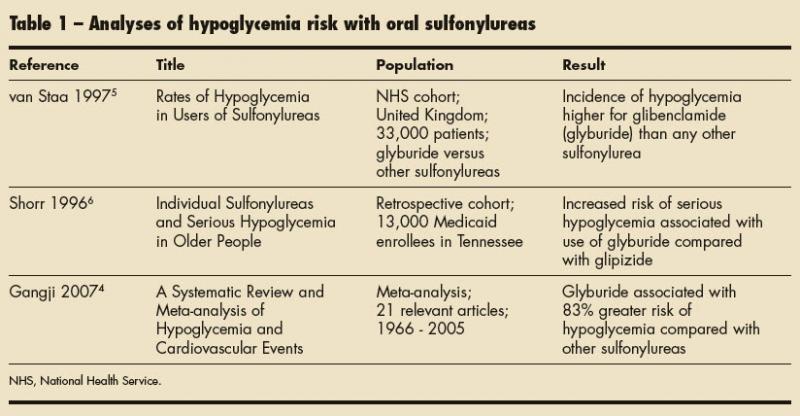
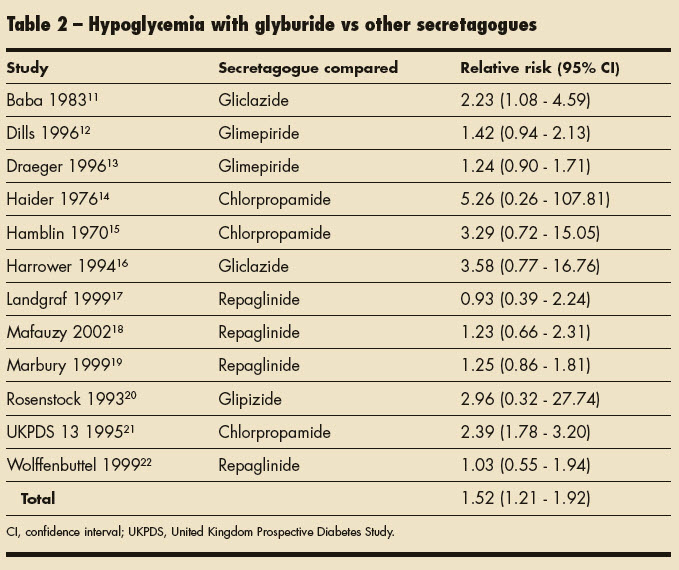
van Staa and associates5 theorized that hypoglycemia risk varies among individual sulfonylureas. They conducted an epidemiological study to evaluate the risk of hypoglycemia in patients with type 2 diabetes mellitus who were receiving chlorpropamide; tolbutamide; glyburide; glipizide; or gliclazide, which is not available in the United States. This study revealed that the risk of hypoglycemia was higher in patients who used glyburide than in those who used other sulfonylureas. Table 2 shows the relative risk of hypoglycemia associated with the available sulfonylureas. van Staa and associates5 also found that patients older than 65 years were at higher risk for hypoglycemia than their younger counterparts.
A number of factors predispose elderly patients to hypoglycemia, including polypharmacy, intercurrent illness, and declining renal and hepatic function.6 This predisposition makes the use of glyburide even more hazardous in elderly patients. Shorr and colleagues6 conducted a retrospective cohort study to assess the risk of sulfonylurea-associated hypoglycemia specifically in the elderly population. The medications reviewed included first-generation agents chlorpropamide, tolazamide, tolbutamide, and acetohexamide, and second-generation agents glyburide and glipizide. This study showed that the lowest risk of hypoglycemia was observed with glipizide, tolbutamide, and tolazamide and the highest risk was seen with chlorpropamide and glyburide.6 A comparison of the rates of hypoglycemia associated with second-generation sulfonylureas revealed that hypoglycemia occurred twice as frequently in patients receiving glybu ride as in those receiving glipizide.6
In a 2007 meta-analysis, glyburide was compared with other antidiabetic medications, including insulin, to determine whether it was associated with a higher risk of hypoglycemia. 4 The meta-analysis, which included 21 studies, revealed a 52% higher risk of hypoglycemia in patients receiving glyburide compared with those taking other insulin secretagogues. In addition, glyburide posed an 83% higher risk of hypoglycemia compared with other sulfonylureas.
Of note, HbA1c values were not significantly different between patients receiving glyburide and those receiving other sulfonylureas.4 This finding indicates that the higher risk of hypoglycemia seen with glyburide is not associated with lower HbA1c values.
DOES GLYBURIDE HAVE A NICHE?
With numerous studies pointing to an increased risk of adverse effects with the use of glyburide, we are left with the question of whether the medication should be removed from the market. Arguments could be made for such an action; however, glyburide has been suggested as a therapeutic option for one set of patients typically treated only with insulin: women with gestational diabetes mellitus. Glyburide is recommended for women with gestational diabetes mellitus who may not necessarily require insulin but who are unable to reach their blood glucose goals with medical nutrition therapy. 7,8 These recommendations are based largely on a randomized unblinded trial that compared the use of glyburide with that of insulin in women with gestational diabetes mellitus. This study found no difference in rates of glycemic control between the two treatment groups.9
Although glyburide has been used in women with gestational diabetes mellitus, it does not have FDA approval for this indication and larger studies are needed to confirm its efficacy and safety in this population. 8 It would also be prudent to conduct studies evaluating the use of other second-generation sulfonylureas, such as glipizide or glimepiride, in women with gestational diabetes mellitus.
GLYBURIDE: NOT RECOMMENDED
The ADA includes a footnote to their treatment algorithm recommending the use of any sulfonylurea other than glyburide or chlorpropamide. 1 Glyburide, glipizide, and gliglimepiride are inexpensive and readily available as generics on most $4 prescription programs. These second-generation sulfonylureas all have similar durations of action; however, they have markedly different relative risks of hypoglycemia. 6,10 This could be explained by glyburide’s increased effect on hepatic insulin sensitivity through high affinity for the ß-cell sulfonylurea receptor, accumulation of active metabolites, and general accumulation in the islet ß-cell, which causes insulin release even after the medication is stopped.4,6,10
It follows that patients who are receiving glyburide, excluding women with gestational diabetes mellitus, should be switched to an equivalent dose of glipizide or glimepiride. In light of evidence that suggests glyburide is associated with at least a 50% higher risk of hypoglycemia than other sulfonylureas, it may be wise to avoid glyburide in all patients who have diabetes.4
1. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2008;31:1-11.
2. Burge M, Schmitz-Fiorentino K, Fischette C, et al. A prospective trial of risk factors for sulfonylurea- induced hypoglycemia in type 2 diabetes mellitus. JAMA. 1998;279:137-143.
3. Scheen AJ, Lefèbvre PJ. Oral antidiabetic agents. A guide to selection. Drugs. 1998;55:225-236.
4. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
5. van Staa T, Abenhaim L, Monette J. Rates of hypoglycemia in users of sulfonylureas. J Clin Epidemiol. 1997;50:735-741.
6. Shorr RI, Ray WA, Daugherty JR, Griffen MR. Individual sulfonylureas and serious hypoglycemia in older people. J Am Geriatr Soc. 1996;44:751-755.
7. American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(suppl 1): S88-S90.
8. Cheung NW. The management of gestational diabetes. Vasc Health Risk Manag. 2009;5: 153-164.
9. Langer O, Conway DL, Berkus MD, et al. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000; 343:1134-1138.
10. Szoke E, Gosmanov NR, Sinkin JC, et al. Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism. 2006;55:78-83.
11. Baba S, Nakagawa S, Takebe K, et al. Comparison of gliclazide and glibenclamide treatment in non-insulin-dependent diabetes. Tohoku J Exp Med. 1983;141(suppl):693-706.
12. Dills DG, Schneider J. Clinical evaluation of glimepiride versus glyburide in NIDDM in a double- blind comparative study. Glimepiride/Glyburide Research Group. Horm Metab Res. 1996;28:426-429.
13. Draeger KE, Wernicke-Panten K, Lomp HJ, et al. Long-term treatment of type 2 diabetic patients with the new oral antidiabetic agent glimepiride (Amaryl): a double-blind comparison with glibenclamide. Horm Metab Res. 1996;28:419-425.
14. Haider Z, Obaidullah S, Fayyaz UD. Comparative study of glibenclamide and chlorpropamide in newly diagnosed maturity onset diabetics. J Pak Med Assoc. 1976;26:23-26.
15. Hamblin JJ, Ismay G, Good MS, et al. A comparative study of glibenclamide and chlorpropamide. (Preliminary report). Postgrad Med J. 1970;(suppl):
92-94.
16. Harrower AD. Comparison of efficacy secondary failure rate, and complications of sulfonylureas. J Diabetes Complications. 1994;8:201-203.
17. Landgraf R, Bilo HJ, Müller PG. A comparison of repaglinide and glibenclamide in the treatment of type 2 diabetic patients previously treated with sulphonylureas. Eur J Clin Pharmacol. 1999;55: 165-171.
18. Mafauzy M. Repaglinide versus glibenclamide treatment of type 2 diabetes during Ramadan fasting. Diabetes Res Clin Pract. 2002;58:45-53.
19. Marbury T, Huang WC, Strange P, Lebovitz H. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract. 1999;43:155-166.
20. Rosenstock J, Carrao PJ, Goldberg RB, Kilo C. Diabetes control in the elderly: a randomized, comparative study of glyburide versus glipizide in non-insulin-dependent diabetes mellitus. Clin Ther. 1993;15:1031-1040.
21. United Kingdom Prospective Diabetes Study (UKPDS). 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83-88.
22. Wolffenbuttel BH, Landgraf R. A 1-year multicenter randomized double-blind comparison of repaglinide and glyburide for the treatment of type 2 diabetes. Dutch and German Repaglinide Study Group. Diabetes Care. 1999;22:463-467.


