Why Are This Boy and His Dad Squinting?
A 15-month-old boy who had recently moved to Guam from a smaller Micronesian island presented for evaluation of cold symptoms. During the physical examination, he was noted to be squinting bilaterally. His mother stated that he had been squinting since birth. A medical provider visiting their island had advised her to have the boy seen by an ophthalmologist after 1 year of age. Additional family history revealed that the boy’s father and a sister have similar vision problems, and that his father had received a diagnosis of color blindness.
Developmental history revealed normal gross motor function for age (eg, walking without assistance), but he would hold his arms extended and hands outward while moving, as if to feel his way. His mother disclosed that he seemed more comfortable moving around at night. He also demonstrated a delay in fine motor control (eg, unable to pick up small objects with a pincer grasp). He had normal speech development, with 5 distinctive words and frequent babbling.

On physical examination, his weight, length, and head circumference were within normal limits for his age. He had no overt dysmorphic facial features. He squinted throughout the encounter and resisted a direct eye examination, but he was noted to have normal appearing orbits, reactive pupils, normal red reflexes bilaterally, and persistent bilateral horizontal nystagmus. Conjunctivae and sclerae appeared normal with no injection, tearing, or ocular exudate. Muscle mass and tone and neurologic reflexes were normal, with no focal deficits. The rest of the physical examination findings were unremarkable.
The boy is the third child of nonconsanguineous parents. His maternal and paternal lineages are Pingelapese, from the Pingelap atoll in Micronesia. He has 2 older full siblings, one of whom is the affected sister, and a maternal half-brother, who is unaffected. None of the child’s mother’s relatives are reported to have any vision defects or nystagmus. Both paternal grandparents are unaffected.
What’s behind this father and son’s photophobia?
Answer: Achromatopsia

A 15-month-old boy who had recently moved to Guam from a smaller Micronesian island presented for evaluation of cold symptoms. During the physical examination, he was noted to be squinting bilaterally. His mother stated that he had been squinting since birth. A medical provider visiting their island had advised her to have the boy seen by an ophthalmologist after 1 year of age. Additional family history revealed that the boy’s father and a sister have similar vision problems, and that his father had received a diagnosis of colorblindness.
Developmental history revealed normal gross motor function for age (eg, walking without assistance), but he would hold his arms extended and hands outward while moving, as if to feel his way. His mother disclosed that he seemed more comfortable moving around at night. He also demonstrated a delay in fine motor control (eg, unable to pick up small objects with a pincer grasp). He had normal speech development, with 5 distinctive words and frequent babbling.
On physical examination, his weight, length, and head circumference were within normal limits for his age. He had no overt dysmorphic facial features. He squinted throughout the encounter and resisted a direct eye examination, but he was noted to have normal appearing orbits, reactive pupils, normal red reflexes bilaterally, and persistent bilateral horizontal nystagmus. Conjunctivae and sclerae appeared normal with no injection, tearing, or ocular exudate. Muscle mass and tone and neurologic reflexes were normal, with no focal deficits. The rest of the physical examination findings were unremarkable.
A detailed, 3-generation pedigree was obtained (Figure). The proband is the third child of the nonconsanguineous union of his unaffected mother and affected father. His maternal and paternal lineages are Pingelapese, from the Pingelap atoll in Micronesia. He has 2 older full siblings, one of whom is the affected sister, and a maternal half-brother, who is unaffected. None of the child’s mother’s relatives are reported to have any vision defects or nystagmus. Both paternal grandparents are unaffected.
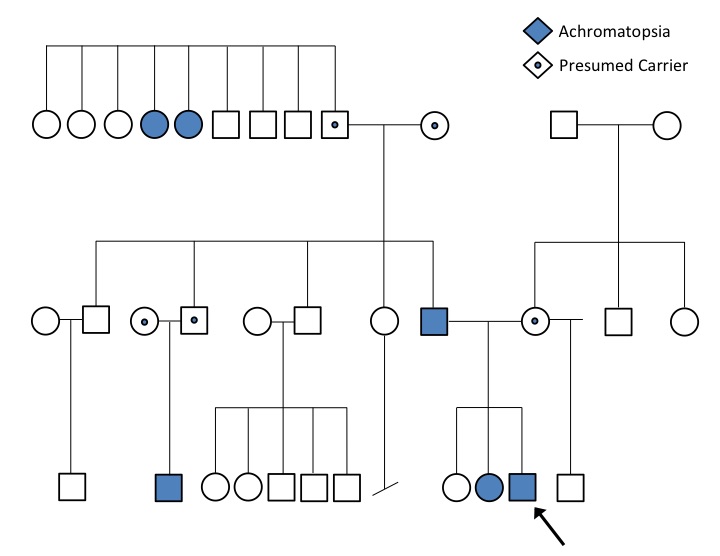
Results of a comprehensive ophthalmologic evaluation showed no abnormalities on either slit-lamp or fundoscopic examination. Nevertheless, a presumptive diagnosis of achromatopsia was made based on the patient’s symptoms and his Pingelapese ancestry. Recommendations were for the boy to wear sunglasses and to return for follow-up in 1 year.
Clinical management of the boy’s case has been mostly supportive. The family has become accustomed to the challenges of the disease process and was not interested in seeking more intensive evaluation or genetic testing. The boy was given glasses with red-filtered lenses for inside use and dark sunglasses for outside use. Yearly follow-up with an ophthalmologist to obtain corrective lens prescriptions was recommended. The family was directed to Internet-based support groups.
Discussion
Intermittent photophobia can be a nonspecific finding in an infant or young child. Persistent photophobia, however, can signal a significant medical problem such as meningitis, glaucoma, or corneal disease. A child with early-onset photophobia that is not progressive is more likely to have corneal or retinal disease.
Achromatopsia is an uncommon cause of photophobia, with nystagmus starting in infancy. The condition attracted wide attention from Oliver Sacks’ book, The Island of the Colorblind, part of which was dedicated to achromatopsia on the Micronesian atoll of Pingelap.1 Although the general prevalence of this severe form of colorblindness is uncommon (from 1 in 30,000 to 1 in 50,000), on this island 5% of the population is affected,2 with approximately 30% being carriers.1
The high prevalence of the disease on Pingelap has been attributed to a narrow genetic pool. In 1775, a typhoon devastated the island, killing 90% of the island’s population and destroying most of island’s resources.3 The population was reduced to approximately 20 people. The hereditary king of the island was the major contributor to the repopulation of the island and is believed to have been a carrier of the condition.4
Vision is a complex process that begins with light reflecting off an object and entering the eye through the clear cornea. The rays pass through the pupil and lens and are focused onto the retina. The retina contains millions of light-sensing nerve cells called cones and rods, aptly named for their 3-dimensional structure. Cones are concentrated in the center of the eye (the macula) and are responsible for clear, sharp, central vision and color differentiation. Outside the macula, rods predominate and provide peripheral vision, motion sensing, and the ability to see in dim light.
Cones are subdivided according to their efficiency at absorbing light in the visual spectrum, 380 nm to 730 nm. S cones (short wavelength, or blue), M cones (medium wavelength, or green), and L cones (long wavelength, or red) absorb maximally at 420 nm, 530 nm, and 560 nm, respectively.5
Classic colorblindness, which affects approximately 7% to 8% of the U.S. male population,6 is characterized by a lack of red-green differentiation and is inherited in a X-linked manner, with a locus identified on band Xq28. In this condition, 1 of the 3 cone pigments is absent, and vision is reduced to the remaining functioning pigments.
Achromatopsia, however, results from a deficiency of all 3 cone types, leading to an absence of not only color vision but also visual acuity in lighted conditions, hence the term day blindness. The majority of affected persons have “complete” achromatopsia, without any discernible cone function; persons with “incomplete” achromatopsia have some residual cone function with some preservation of visual acuity.
Achromatopsia is inherited in an autosomal recessive manner. To date, 4 genes have been implicated: GNAT2, PDE6C, CNGA3, and CNGB3. GNAT2 encodes the ɑ subunit of the cone protein transducin, and PDE6C encodes the ɑ subunit of the cone protein phosphodiesterase. CNGA3 and CNGB3, mutations of which are implicated in the majority of achromatopsia cases,7 are involved in the proper development of the ɑ (CNGA3) and β (CNGB3) subunits of nucleotide-gated channels in the conal phototransduction cascade.7-9 CNGB3 is the gene affected among the Pingelapese. Complete loss of function of CNGB3 results from an amino acid change of serine to phenylalanine at a highly conserved region of the gene.8
Symptoms of complete achromatopsia include low visual acuity, nystagmus, photophobia, and the subsequent development of cataracts.10 The degree of myopia typically is less than 20/200; initial symptoms in infants typically include nystagmus and squinting, as was the case with our patient.
There is no effective treatment for persons with achromatopsia. Symptoms can be treated with particularly tinted lenses or avoidance of daytime activities.11 Leber congenital amaurosis has been a model of a disease that has been successfully treated with retinal injections of an adeno-associated virus (AAV) designed for RPE65.12,13 Canine and murine models also have been developed for AAV treatment of achromatopsia, and several studies have shown promising results.7,14-16 Mouse knockout models have been developed specifically for GNAT2 and CNGA3 mutations, and young canine models have been developed for CNGB3. In the latter, subretinal injections of AAV-mediated gene have resulted in improved electroretinography results for more than a year afterward.11 Further studies improved upon the age restrictions of the canine model; Komáromy and colleagues demonstrated a restoration of cone function in dogs older than 1 year of age after combining AAV gene therapy with ciliary neurotrophic factor (CNTF).16 CNTF improves the growth of neurotransmitters and ultimately allows for improved retinal function.
While achromatopsia is a rare disorder in the general population, the condition occurs with greater frequency in certain ethnicities and certain areas of the world and should be considered in the differential diagnosis of vision abnormalities in an infant or toddler.
As in this case, knowledge about local incidence rates of endemic diseases guides differential diagnosis and treatment strategies in specific communities (eg, a high methicillin-resistant Staphylococcus aureus incidence affects antibiotic choice; a high Lyme disease incidence suggests the most likely cause of Bell palsy).
David Allen Austin, MD, is a pediatrician at IHP Medical Group in Dededo, Guam, and a community faculty member in the Department of Pediatrics at Eastern Virginia Medical School in Norfolk, Virginia.
Samantha Schrier Vergano, MD, is an assistant professor in the Department of Pediatrics at Eastern Virginia Medical School and an attending physician in the Department of Pediatrics, Division of Medical Genetics and Metabolism, at Children’s Hospital of The King’s Daughters in Norfolk, Virginia.
John W. Harrington, MD, is the division director of General Academic Pediatrics at Children’s Hospital of The King’s Daughters and a professor of pediatrics at Eastern Virginia Medical School in Norfolk, Virginia.
References
1. Sacks O. The Island of the Colorblind. New York, NY: Vintage: 1998.
2. Carr RE, Morton NE, Siegel IM. Achromatopsia in Pingelap Islanders: study of a genetic isolate. Am J Ophthalmol. 1971;72(4):746-756.
3. Sheffield VC. The vision of Typhoon Lengkieki. Nat Med. 2000;6(7):746-747.
4. Hussels IE, Morton NE. Pingelap and Mokil atolls: achromatopsia. Am J Hum Genet. 1972;24(3):304-9.
5. Heywood CA, Kentridge RW. Achromatopsia, color vision, and cortex. Neurol Clin. 2003;21(2):483-500.
6. Bennett J. Gene therapy for color blindness. N Engl J Med. 2009;361(25): 2483-2484.
7. Komáromy AM, Alexander JJ, Rowlan JS, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;10(295);2581-2593.
8. Sundin OH, Yang J-M, Li Y, et al. Genetic basis of total colourblindness among the Pingelapese islanders. Nat Genet. 2000;25(3):289-293.
9. Deeb SS. Molecular genetics of colour vision deficiencies. Clin Exp Optom. 2004;87(4-5):224-229.
10. Brody JA, Hussels I, Brink E, Torres J. Hereditary blindness among Pingelapese people of Eastern Caroline Islands. Lancet. 1970;295(7659):1253-1257.
11. Pang J-j, Alexander J, Lei B, et al. Achromatopsia as a potential candidate for gene therapy. Adv Exp Med Biol. 2010;664:639-646.
12. Pang J-j, Chang B, Kumar A, et al. Gene therapy restores vision-dependent behavior as well as retinal structure and function in a mouse model of RPE65 Leber congenital amaurosis. Mol Ther. 2006;13(3): 565-571.
13. Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979-990.
14. Michalakis S, Mühlfriedel R, Tanimoto N, et al. Restoration of cone vision in the CNGA3-/- mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18(12):2057-2063.
15. Pang J-j, Deng W-T, Dai X, et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS One. 2012;7(4):e35250.
16. Komáromy AM, Rowlan JS, Corr ATP, et al. Transient photoreceptor deconstruction by CNTF enhances rAAV-mediated cone functional rescue in late stage CNGB3-achromatopsia. Mol Ther. 2013;21(6):1131-1141.
Disclosure
Samantha Schrier Vergano, MD, is on the clinical advisory board of Ambry Genetics.
Acknowledgement
The authors thank Brooke Spangler, MS, CGC, for her help creating the pedigree.


