Septic Arthritis, or Something More?: Leukemia Presenting as Joint Pain and Swelling
ABSTRACT: The differential diagnosis for a child with a painful and swollen joint is broad and includes trauma, infection-related diseases (such as septic arthritis or reactive arthritis), rheumatologic disorders (such as juvenile idiopathic arthritis), and malignancy. Acute lymphoblastic leukemia (ALL) can have a vague presentation; still, a significant percentage of children with it have musculoskeletal symptoms such as bone pain, arthralgia, myalgia, and, rarely, leukemic arthritis—the latter of which might be misidentified as septic arthritis.
A 5-year-old boy presented with a 3-day history of right knee pain, swelling, and a refusal to bear weight. He had a history of migratory joint pain, and he had been hospitalized 2 months prior with a presumed septic left elbow with osteomyelitis. The patient’s pain was interrupting sleep and had not been relieved by ibuprofen.
A review of systems was positive for mild fatigue but negative for fevers, weight loss, headaches, dizziness, rash, easy bleeding or bruising, or epistaxis. He was currently taking oral clindamycin as treatment for the presumed septic left elbow and osteomyelitis.
Past Medical History
A review of the patient’s medical record revealed numerous emergency department (ED) visits over the past 4 months for extremity and joint pain. At an ED visit 3 months prior to admission, the patient had been diagnosed with an occult fracture of the lateral condyle of the right humerus (Figure 1). At the time of casting, a complete blood count (CBC) demonstrated a low absolute neutrophil count, mild anemia, and a low platelet count. The patient was admitted to the hospital for further workup of pancytopenia; however, follow-up laboratory analysis the next morning showed a completely normal CBC, prompting the patient to be discharged home.

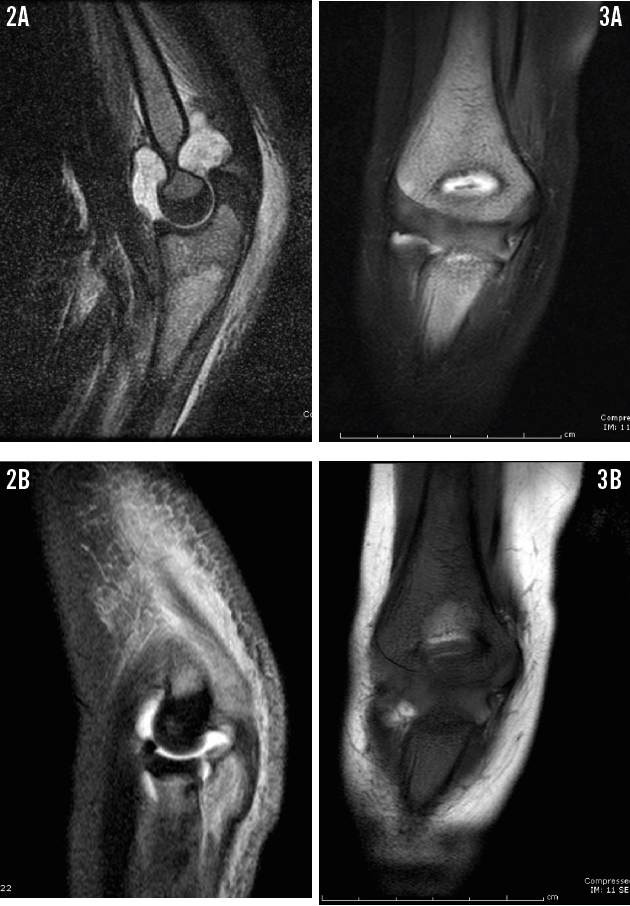
Over the next month, now 2 months before the current presentation, his pain continued. He was admitted for left elbow pain and significant joint swelling concerning for a septic joint. Joint aspirate testing revealed a white blood cell (WBC) count of 111,670/µL (96% neutrophils) and negative culture results. T2-weighted magnetic resonance imaging (MRI) of the left elbow showed increased marrow signal at the proximal radius, ulna, and metaphysis of the medial humeral condyle (Figure 2), which prompted a diagnosis of septic left elbow with osteomyelitis. However, given the boy’s history of recurrent joint pain and his recent fracture, a diagnosis of chronic recurrent multifocal osteomyelitis (CRMO) also was strongly considered. MRI of the right elbow also was performed at that time, but with limited results due to motion artifact (Figure 3). The patient was started on intravenous clindamycin and underwent irrigation and debridement of the joint. He was discharged home on postoperative day 8 after placement of a central venous catheter for home antibiotic infusion.
The patient followed up with the orthopedics and infectious disease clinics at 2 weeks and 4 weeks postoperatively, and he had an uneventful recovery and normal laboratory evaluation results. At 4 weeks, intravenous clindamycin was switched to oral clindamycin for the final 3 weeks of antibiotic therapy.
Two weeks prior to the current presentation, the boy returned to the ED twice—once for left wrist pain and once for right clavicle pain. Again, no abnormalities or fractures were noted on right shoulder and left wrist radiographs. A CBC obtained at the most recent ED visit 1 week prior to admission was notable for a WBC count of 11,300/µL with 3% blasts, 3% metamyelocytes, and 3% myelocytes, along with a hemoglobin level of 11.8 g/dL and a platelet count of 393 × 103/µL. Results of radiographs were normal. A consulting oncologist attributed the blast cells seen on the CBC to recovering marrow from CRMO or a recent serious infection such as septic joint with osteomyelitis.
In the setting of recent culture-negative septic arthritis and numerous prior visits for joint pain, the patient now presented with right knee pain and refusal to bear weight.
Physical Examination
Physical examination revealed a well-appearing boy, who was afebrile and in no acute distress, with right knee swelling and tenderness to palpation. Vital signs were as follows: weight, 21 kg; height, 114 cm; temperature, 37.4°C; heart rate, 146 beats/min; and blood pressure, 111/72 mm Hg.
The child was normocephalic and atraumatic, his oropharynx was clear without enlarged tonsils or pharyngeal exudates, and his mucous membranes were moist. Tympanic membranes were clear without effusion. Pupils were equal, round, and reactive to light; extraocular movements were full and intact and free of nystagmus. The neck was supple, without thyromegaly or lymphadenopathy, and the trachea was on the midline.
The lungs were clear to auscultation bilaterally, without wheezes, rales, or rhonchi. Chest examination revealed no palpable masses or axillary lymphadenopathy. Cardiovascular examination revealed normal S1 and S2 and regular rate and rhythm without murmurs, heaves, rubs, thrills, or gallops. Abdominal examination showed a nontender, nondistended abdomen without hepatomegaly or splenomegaly, no abdominal masses, and normal active bowel sounds. Genitourinary examination revealed normal external male genitalia, no inguinal lymphadenopathy, and normal size testes descended into the scrotal sac.
Examination of the extremities and musculoskeletal examination revealed right knee tenderness to palpation with swelling, and limited passive and active range of motion secondary to pain, but no erythema or warmth over the joint. The upper extremities and shoulders were symmetric without tenderness to palpation or swelling, and full range of motion.
Neurologic examination findings were nonfocal with normal muscle strength, tone and sensation, and intact cranial nerves. Gait examination was limited due to right knee pain, but no ataxia was demonstrated on cerebellar function testing, and deep tendon reflexes were 2+ throughout. The skin examination demonstrated no rashes or cyanosis, capillary refill was less than 2 seconds, and no edema was seen other than that over the right patella.
Diagnostic Testing
Joint aspiration of the right knee was attempted twice by orthopedic surgery; however, no joint fluid could be obtained. The patient was started on intravenous vancomycin and ceftriaxone out of concern for hematologic spread of his septic joint while on oral clindamycin.
Results of a CBC showed a WBC count of 8,100/µL, a hemoglobin level of 10.2 g/dL, and a platelet count of 380 × 103/µL. Manual differential showed 50% segmented neutrophils, 26% lymphocytes, 9% monocytes, 3% metamyelocytes, 1% myelocytes, and 7% blast cells. Blood chemistry results were remarkable for a lactate dehydrogenase (LDH) level of 2,143 U/L and elevated liver transaminases (aspartate aminotransferase, 196 U/L; and alanine aminotransferase, 542 U/L). The oncology service was consulted to obtain bone marrow aspiration and biopsy, the results of which were consistent with acute lymphoblastic leukemia (ALL), with significant sections of bone marrow replaced by blast cells (Figure 4). Cytogenetic analysis of the boy’s cultured bone marrow cells demonstrated a highly aberrant karyotype, 57,XY,+X,+X,+6,+10,+11,+12,+13,+20,+21,+21,+22[4]/46,XY[22] (Figure 5).

The patient was diagnosed with pre–B-cell ALL following bone marrow biopsy and was transferred to the oncology floor for induction chemotherapy and further workup, including lumbar puncture and initiation of intrathecal chemotherapy with cytarabine.
After being transferred to the oncology service, the patient was started on induction chemotherapy (Children’s Oncology Group trial for standard risk B-precursor ALL protocol, COG-AALL0932) with dexamethasone, vincristine, pegaspargase, and intrathecal methotrexate. He responded well with resolution of joint symptoms and no severe complications. Bone marrow aspirate at the end of induction chemotherapy demonstrated morphologic remission. The patient remains in remission and is undergoing maintenance chemotherapy per the COG-AALL0932 protocol.

Discussion
The differential diagnosis for a child with a painful and swollen joint is broad and includes trauma, infection-related diseases (such as septic arthritis or reactive arthritis), rheumatologic disorders (such as juvenile idiopathic arthritis), and malignancy. A history of migratory arthritis is helpful in narrowing the differential diagnosis to juvenile idiopathic arthritis, connective tissue disease, acute rheumatic fever, Lyme disease, CRMO, and malignancy. In our patient, the presence of peripheral blasts and an elevated LDH level guided the diagnosis to ALL with the unusual presentation of a leukemic joint.
This case demonstrates the sometimes nebulous presentation of ALL. Classically, a child may present with fever, limp, bone pain, fatigue, pallor, petechiae, hepatosplenomegaly, anemia, weight loss, anorexia, and/or headache. While a history of bone pain often is a tip-off to the diagnosis, our patient’s presentation was confounded by a previous presentation of “septic arthritis.” Although the boy’s joint aspirate was sterile, a WBC count of more than 100,000/µL with a neutrophil predominance is consistent with infection.
Additionally, negative joint aspirate cultures do not necessarily rule out infection, since they may be negative in 35% of cases of bacterial arthritis.1 It is important to note, however, that septic arthritis most commonly affects larger joints such as the hips or knees. Septic arthritis of the elbow is rare.
Approximately 21% to 59% of children with ALL are reported to have musculoskeletal complaints at the time of diagnosis, and they often are referred to orthopedic surgeons and rheumatologists for further workup.2 Musculoskeletal symptoms of ALL include bone pain, arthralgia, myalgia, and, rarely, leukemic arthritis, as was the case in our patient.
The pathophysiology of bone pain in children with ALL is thought to be secondary to rapid proliferation of malignant cells in the bone marrow; however, the pathogenesis of leukemic arthritis is less well understood. Most experts attribute the arthritis to a local reaction to bony, periosteal, or capsular infiltration by malignant cells.3-5 Nevertheless, some authors have described infiltrative findings on synovial biopsy with evidence of leukemic cells in patients with leukemic arthritis.6 Corresponding with most reported cases in the literature, our patient had no blasts in his joint aspirate.

Leukemic arthritis is characterized by a painful, often swollen or erythematous joint. Effusion may be present, as was the case in our patient, or there may be soft tissue swelling caused by synovial inflammation. The pain is notably disproportionate to the degree of arthritis, a finding that may help distinguish leukemic arthritis from a rheumatologic process. The arthritis may be monoarticular or polyarticular and typically involves the extremities. When the axial skeleton is involved, the most commonly affected joints are the vertebrae, which often have compression fractures. The pain of a leukemic joint typically resolves with treatment (ie, chemotherapy) of the underlying condition. Similarly, case reports have found that patients’ joint symptoms return during relapses.7
Clues that can lead to the diagnosis of ALL include nocturnal pain, pain that is poorly controlled with nonsteroidal anti-inflammatory drugs, and the presence of systemic symptoms such as fatigue, anorexia, weight loss, fevers, and pallor. Routine blood tests such as a CBC can aid in identifying cytopenia or peripheral blast cells, although CBC results may be normal. In fact, one retrospective study showed that patients with childhood leukemia presenting with predominantly musculoskeletal complaints were more likely to have normal CBC results.8 In children whose CBC analysis does not demonstrate peripheral blasts or cytopenia, an elevated LDH level is helpful in distinguishing infectious or rheumatologic etiologies from ALL.9 Other studies have shown that elevated inflammatory markers in the absence of leukocytosis or thrombocytosis may signify an infiltrative process in the bone marrow.10,11
ALL can present with a number of radiographic changes. The numerous radiographs taken of our patient’s extremities showed none of the commonly described bony changes. These typical changes include metaphyseal bands, periosteal reaction, osteopenia, lytic lesions, sclerosis, pathologic fractures, and effusions. Classically, leukemic marrow presents as increased brightness on fat-suppressed T2-weighted MRI.12 These changes can be seen in both ALL and osteomyelitis, which confounded the initial diagnosis in our patient.
Adriana Hernandez, MD, is a second-year resident at Children’s Hospital Los Angeles Pediatric Residency Program in Los Angeles, California.
Alexander Van Speybroeck, MD, is an assistant professor of pediatrics at Keck School of Medicine of the University of Southern California and a pediatrician at Children’s Hospital Los Angeles.
References
1. Krogstad P. Septic arthritis. In: Cherry JD, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez P, eds. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. Vol 1. 7th ed. Philadelphia, PA: Elsevier Saunders; 2014:727-734.
2. Heinrich SD, Gallagher D, Warrior R, Phelan K, George VT, MacEwen GD. The prognostic significance of the skeletal manifestations of acute lymphoblastic leukemia of childhood. J Pediatr Orthop. 1994;14(4):105-111.
3. Chell J, Fernandes JA, Bell MJ. The orthopaedic presentation of acute leukaemia in childhood. Ann R Coll Surg Engl. 2001;83(3):186-189.
4. Evans TI, Nercessian BM, Sanders KM. Leukemic arthritis. Semin Arthritis Rheum. 1994;24(1):48-56.
5. Riccio I, Marcarelli M, Del Regno N, et al. Musculoskeletal problems in pediatric acute leukemia. J Pediatr Orthop B. 2013;22(3):264-269.
6. Spilberg I, Meyer GJ. The arthritis of leukemia. Arthritis Rheum. 1972;15(6): 630-635.
7. Bradlow A, Barton C. Arthritic presentation of childhood leukaemia. Postgrad Med J. 1991;67(788):562-564.
8. Jonsson OG, Sartain P, Ducore JM, Buchanan GR. Bone pain as an initial symptom of childhood acute lymphoblastic leukemia: association with nearly normal hematologic indexes. J Pediatr. 1990;117(2 pt 1):233-237.
9. Wallendal M, Stork L, Hollister JR. The discriminating value of serum lactate dehydrogenase levels in children with malignant neoplasms presenting as joint pain. Arch Pediatr Adolesc Med. 1996;150(1):70-73.
10. Cabral DA, Tucker LB. Malignancies in children who initially present with rheumatologic complaints. J Pediatr. 1999;134(1):53-57.
11. Kobayashi D, Satsuma S, Kamegaya M, et al. Musculoskeletal conditions of acute leukemia and malignant lymphoma in children. J Pediatr Orthop B. 2005;14(3):156-161.
12. Orth RC, Guillerman RP. Skeletal manifestations of systemic disease. In: Coley BD, ed. Caffey’s Pediatric Diagnostic Imaging. Vol 2. 12th ed. Philadelphia, PA: Elsevier Saunders; 2013:1543-1560.


