Conditions That Might Affect the Brain
Traumatic Brain Injury and Fecal Retention
Jon O. Ebbert, MD, and Timothy J. Young, MD
A 31-year-old man with a history of traumatic brain injury was hospitalized because of failure to thrive, constipation, and intermittent diarrhea with soiling.
A palpable lower abdominal mass was noted. A CT scan of the abdomen and pelvis revealed retained stool in the rectum, a rectosigmoid colon dilated to 15 cm (long arrow), and compression of the bladder (short arrow).

Regular bowel care is a fundamental but frequently overlooked component in the care of patients with CNS dysfunction. Blunted rectal sensation may account for a lack of the desire to defecate. Soiling may occur because rectal distention elicits internal sphincter relaxation at a lower threshold, while external sphincter contraction is impaired. Impaired colonic response to a meal may also contribute to constipation.
In this patient, docusate with benzocaine enemas induced large bowel movement. The patient was discharged with an aggressive bowel regimen. At follow-up, he reported normal daily bowel movements. ■
Neurocysticercosis
Alexander M. Sy, MD, and Shobahana Chaudhari, MD
Metropolitan Hospital Center, New York
An 18-year-old woman from Mexico was hospitalized because of severe headache with nausea and vomiting. Her headaches had started 4 years earlier and had progressively worsened. They occurred mainly in the occipital region and were pulsating, worse on bending down, and unrelieved by any medication. They were often accompanied by dizziness and presyncope.
The patient’s vital signs were stable. Funduscopy revealed papilledema. Physical and neurological findings were otherwise normal. Initial laboratory results showed a white blood cell count of 12,340/µL with left shift. Results from a serum chemistry panel, hepatic panel, and chest radiograph were normal.

A cranial CT scan showed calcifications (arrows) scattered throughout the brain parenchyma with hydrocephalus. Intravenous ceftriaxone and metronidazole were started; phenytoin was added after seizures developed. Results from blood cultures were negative. The patient underwent a ventriculoperitoneal shunt. Results of cerebrospinal fluid analysis and culture, Cytomegalovirus serology, Toxoplasma serology, and tuberculosis polymerase chain reaction were all negative. Serology for cysticercosis was reactive. Postoperatively, albendazole (800 mg/d) and dexamethasone were started.
Discussion. Neurocysticercosis, the most common parasitic infection of the CNS, is caused by tissue-invading larval forms of the pork tapeworm Taenia solium.1 The infection is more common among immigrants from Latin America, Asia, and Africa. Immigration from areas where the disease is endemic has led to an increased incidence in the United States. This is especially true in southwestern California, where seizure caused by neurocysticercosis may account for 10% of emergency department visits.2 In nonborder states, the condition is often underrecognized.
In the CNS, larvae can be deposited anywhere from the brain parenchyma to the spinal cord. Symptoms, such as seizures, focal neurological signs, and intracranial hypertension, arise when an encysted worm dies and the body mounts an associated inflammatory response. However, patients may be asymptomatic.

Neuroimaging may show a nonenhancing hypodense lesion, variable degrees of edema, calcifications, or hydrocephalus. Conditions that can present with similar features include toxoplasmosis, schistosomiasis, tuberculosis, cytomegalovirus infection, abscess, primary brain and metastatic cancers, trichinosis, and sarcoidosis. Finding a scolex as a mural nodule within the cyst is pathognomonic for neurocysticercosis. The appearance on CT or MRI scans is frequently nonspecific and may be difficult to differentiate from other brain lesions. A definitive diagnosis can be made using proposed diagnostic criteria based on clinical presentation, imaging, serology, and epidemiological data.3
Treatment consists of anticonvulsants, antihelminthic therapy (with either albendazole or praziquantel), and corticosteroids. Surgery is reserved for hydrocephalus or giant cysts in the setting of intracranial hypertension. No treatment is required for asymptomatic patients. ■
References:
1. DeGiorgio CM, Medina MT, Durón R, et al. Neurocysticercosis. Epilepsy Curr. 2004;4:107-111.
2. Ong S, Talan DA, Moran GJ, et al; EMERGEncy ID NET Study Group. Neurocysticercosis in radiographically imaged seizure patients in U.S. emergency departments. Emerg Infect Dis. 2002;8:608-613.
3. Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;24;57:177-183.
Malignant Melanoma
Dr. Reynold C. Wong
A previously asymptomatic, large black plaque on a 65-year-old man’s scalp recently began to bleed. The lesion had grown considerably since it first appeared as a small black macule 3 years earlier.

A clinical diagnosis of malignant melanoma was confirmed by an incisional biopsy. An excisional biopsy of the 3.85 mm thick cancer was performed several days later. The patient refused biopsy of clinically palpable lymph nodes in his neck.
Common sites of melanoma metastases include the nodes, lungs, liver, brain, and bone. This patient died 8 months later with metastases to his brain. ■
Young Man with a Numb Chin
Pamela Havlen, MD, and Rachel E. Salas, MD
University of Texas Medical Branch at Galveston
Numb chin syndrome has been described in the literature since the 1960s and has been recognized as a sign of possible serious illness since that time.1 It is defined as a numbness or paresthesia over the territory of the mental nerve, which includes the chin and lower lip.
This syndrome can result from nonmalignant etiologies—such as trauma, drugs, diabetes mellitus, syphilis, amyloidosis, sarcoidosis, sickle cell anemia, and vasculitis, but the most common and most worrisome cause is metastatic malignancy.2,3 Numb chin syndrome has most frequently been associated with breast cancer and lymphoma; however, it has also been linked to many other malignancies.4 Often a marker for recurrence of a known and now widespread malignancy, it has also been the initial presenting symptom of a new and advanced malignancy.3 In fact, Massey and colleagues5 found that in 47% of cases, numb chin syndrome preceded the diagnosis of the primary tumor.

Symptoms in this syndrome are linked to the inferior alveolar nerve. This nerve has only sensory capabilities and innervates the lower lip and chin as well as the lower gingiva and teeth.2 It originates as part of the posterior trunk of the mandibular division of the trigeminal nerve. It enters the mandible at the mandibular foramen and travels along the mandible until it exits at the mental foramen. Upon exit from the mental foramen, the inferior alveolar nerve becomes the mental nerve.2
Thus, the neuropathy has been hypothesized to be caused by several different mechanisms. The possibilities include compression of the inferior alveolar nerve itself by mandibular metastases, mandibular nerve compression by skull-base tumors, leptomeningeal metastases, and tumor cell infiltration of the inferior alveolar nerve sheath.3 Lossos and Siegal4 found in a report of 42 patients with numb chin syndrome that mandibular metastases were the most common cause of this syndrome and leptomeningeal metastases occurred next most commonly. They stated that these 2 etiologies may sometimes be differentiated by the presence of other cranial neuropathies indicating a leptomeningeal origin. Burt and associates2 argued that mandibular metastases are by far the most common cause of numb chin syndrome and if such metastases have not been found, then it is likely they were simply not detectable at that time and by current technology.
In addition to a thorough history and physical examination, the recommended studies in the evaluation of numb chin syndrome include a panoramic radiograph of the mandible, CT of the brain and skull base, MRI, and nuclear bone scintigraphy. If the cause of the patient’s symptoms remains uncertain, cerebral spinal fluid examination may be useful in identifying leptomeningeal metastases.3
Therapy is usually directed at the specific cause, whether it is malignant or benign. In the case of mandibular metastases, systemic therapy should be initiated or changed. Skull-base tumors may respond to local radiation therapy, and leptomeningeal metastases may respond to whole brain irradiation or intrathecal methotrexate injection.4 Unfortunately, the appearance of the numb chin syndrome in the setting of malignancy is a sign of poor prognosis, and treating the syndrome does not improve the outcome.3 Patients with mandibular metastases survive approximately 5 months, and patients with leptomeningeal metastases survive about 12 months.4
Outcome of the case. The patient in this case had already received a diagnosis of metastatic adenocarcinoma that was clearly progressive. An MRI scan of the brain revealed multiple meningeal metastases (Figure). No further investigations of his numb chin syndrome were undertaken. It was believed that the meningeal metastases were the cause of his symptoms, and he started whole brain radiation after completing the local radiation to his epidural metastasis. He may have had a mandibular metastasis, but obtaining this information would not have affected his already poor prognosis. He was referred to hospice care. ■
References:
1. Calverly JR, Mohnac AM. Syndrome of the numb chin. Arch Intern Med. 1963;112:819-821.
2. Burt RK, Sharfman WH, Karp BI, Wilson WH. Mental neuropathy (numb chin syndrome): a harbinger of tumor progression or relapse. Cancer. 1992;70:877-881.
3. Marinella MA. Numb chin syndrome: a subtle clue to possible serious illness. Hospital Physician. 2000;36:54-56.
4. Lossos A, Siegal T. Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology. 1992;42:
1181-1184.
5. Massey EW, Moore J, Schold SC. Mental neuropathy from systemic cancer. Neurology. 1981;31:1277-1281
Elderly Man with Sudden Vision Loss
Leonid Skorin, Jr, DO
A 76-year-old man presents with a sudden severe, painless loss of vision in his left eye. On awakening that day, he became aware of a central black spot in his left field of vision. He noticed a few floaters in the eye but no flashing lights. His right eye does not seem to be affected. He also reports a new onset of generalized head pain and pain on the left side of the jaw after chewing. He has no history of ocular injury or surgery.
The patient has arthritis and diet-controlled hyperlipidemia. He takes methotrexate, hydrocodone, and folic acid. He is allergic to celecoxib and experiences GI upset with noncoated aspirin.
The patient’s corrected visual acuity is 20/30 in the right eye; in the left eye, he has hand motion vision at 5 ft. There is a 2+ afferent pupillary defect in the left eye. Examination of the visual fields by confrontation shows constriction with a central scotoma in the left eye. Extraocular muscle movements are full with no restrictions, and the intraocular pressures are normal.
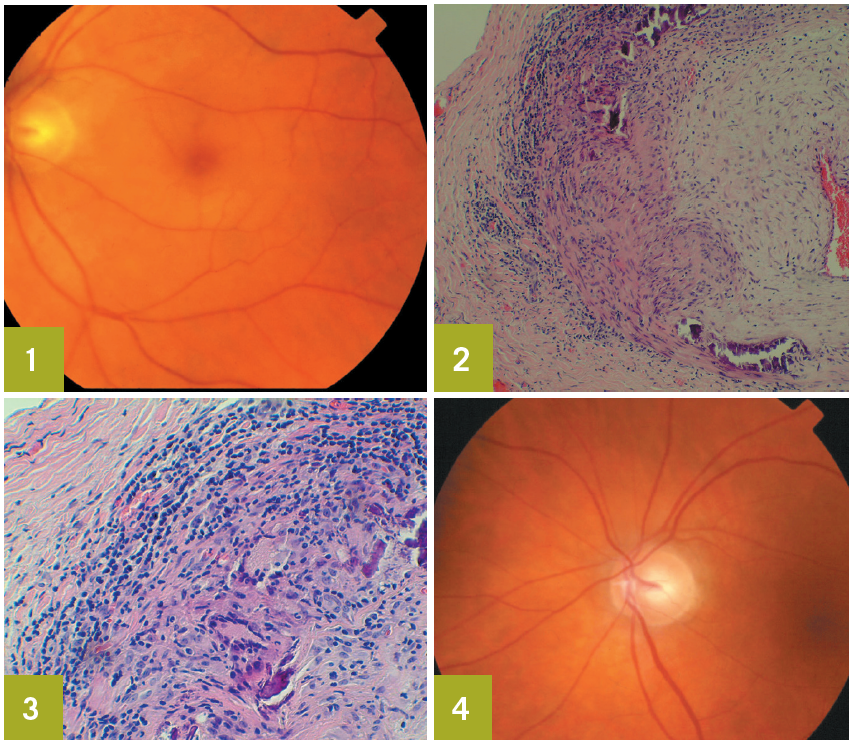
Slitlamp evaluation identifies nuclear sclerotic cataracts in both eyes; the anterior segment is otherwise unremarkable. Funduscopic examination reveals a pallid optic disc with a cherry-red spot in the left macula (Figure 1). The blood vessels are more attenuated in the left retina than in the right. Sludging of red blood cells in the retinal vasculature of the left eye is also seen. There is no retinal detachment. No bruits are heard on auscultation of the carotid arteries.
Ocular massage is performed in the office. A regimen of oral prednisone, 60 mg/d, and coated aspirin, 81 mg/d, is initiated. Results of blood tests obtained the same day show an erythrocyte sedimentation rate (ESR) of 84 mm/h; top normal value for this patient is 76/2 = 38 mm/h. The C-reactive protein (CRP) level is 3.2 mg/dL. The complete blood cell (CBC) count is normal. A carotid duplex ultrasonographic scan on the following day reveals bilateral internal carotid plaque with less than 40% stenosis and normal flow velocities. Because of the elevated ESR and CRP values, a temporal artery biopsy is scheduled.
The biopsy findings include acute and chronic inflammation. There is evidence of thickening of the media, destruction of the internal elastic lamina, and panarteritis (Figures 2 and 3). These findings confirm the diagnosis of temporal arteritis.
The patient continues to take oral prednisone. After 2 months, his headache and jaw claudication have resolved; however, the vision in his left eye has not improved. Optic atrophy eventually develops in that eye (Figure 4). The right eye is not affected. The oral prednisone is being slowly tapered. His ESR and CRP values will be monitored, and he is instructed to report any new symptoms.
Epidemiology. Temporal arteritis, also known as giant cell arteritis and cranial arteritis, is a chronic inflammatory disease that may involve blood vessels that supply the optic nerve, retina, and brain.
Temporal arteritis is the most common primary vasculitis that affects white persons, especially those of Scandinavian and Northern European descent.1 The median age of affected patients is 76 years. Temporal arteritis is more common in women than in men, with respective incidences of 72.6% and 27.4%.2-4
Clinical presentation. Up to 50% of patients report ocular symptoms.5 Between 70% and 80% of patients present with a pallid (chalky white) swollen disc, known as arteritic anterior ischemic optic neuropathy.5 Less common causes of vision loss in persons with temporal arteritis include nonembolic central retinal artery occlusion, such as was seen in this patient. This occlusion may be the presenting sign in up to 14% of patients; it may also occur simultaneously with the optic neuropathy.3,6
Other ocular presentations include amaurosis fugax (31% of patients); eye pain (8.2%); posterior ischemic optic neuropathy (7.1%); and ocular ischemic syndrome (1.2%). About 10% to 15% of patients present with transient diplopia or frank cranial nerve palsy.2,3,7 It is unclear whether the cause of the ophthalmoplegia is neurogenic (peripheral cranial
neuropathy or brain stem ischemia) or myogenic (or bital or muscle ischemia).8
Nonocular findings include fatigue; anorexia; weight loss; polymyalgia rheumatica; altered mental status; headache; scalp tenderness; scalp necrosis; intermittent jaw or tongue claudication; ear, throat, or neck pain; facial edema; nonproductive cough; dysphasia; angina pectoris; congestive heart failure; myocardial infarction; and stroke.8-12
Laboratory tests. These include measurement of ESR (Westergren method) and CRP level and CBC count. Affected patients often have a mild to moderate normochromic-normocytic anemia with a normal leukocyte and differential count.12 Thrombocytosis is seen in up to 50% of patients.13 A markedly elevated ESR (higher than 40 mm/h) is found in about 90% of patients who have biopsy-proven temporal arteritis.12
The ESR is affected by age and sex. The following formulas are useful for estimating the normal upper values: male = age/2, female = (age + 10)/2.11 Because the ESR also tends to become falsely elevated in the presence of anemia, a CBC count is helpful in patients with suspected temporal arteritis.8
The CRP level is unaffected by anemia and does not tend to vary with age.8,11 When both the ESR and CRP level are elevated, the specificity is 97% for the diagnosis of temporal arteritis in the presence of suggestive clinical findings.2
Biopsy. This procedure is performed in all patients with suspected temporal arteritis.12 Temporal artery biopsy is 100% specific and up to 95% sensitive for the disease.14 Verification of the diagnosis by biopsy supports the use of systemic corticosteroid therapy, which often is required for up to a year and may be associated with severe systemic complications.12
Temporal artery biopsy is typically performed unilaterally on the symptomatic side. If the initial biopsy results are negative but clinical suspicion remains high, a biopsy of the contralateral side should be performed.15 There is up to a 5% chance that results of this biopsy will be positive when results of the first biopsy are negative.15,16 A false-negative biopsy may result from discontinuous arterial involvement (intervals of histologically normal-appearing tissue known as “skip lesions”), especially in the setting of an insufficient length of specimen or inadequate sectioning of the histopathologic tissue.7,12
Treatment. Initiation of therapy should not be delayed for performance of the temporal artery biopsy, because the artery remains abnormal for at least 2 weeks after corticosteroid therapy is started.12 Whether the initial treatment should consist of high-dose oral prednisone or intravenous methylprednisolone is still debated. If the patient has not yet lost any vision—as in the case of amaurosis fugax—or if the seeing eye is threatened in a patient who has recently lost vision in one eye as a result of temporal arteritis, intravenous methylprednisolone is recommended.12 This treatment is thought to help prevent the onset of blindness in the seeing eye or further loss of vision in the threatened eye.10,11 Without treatment, contralateral eye involvement develops in 50% to 95% of patients, often within weeks.17,18
The systemic corticosteroids can be tapered slowly as the symptoms and laboratory inflammatory markers return to normal.11 Long-term treatment (months to years) may be necessary in some patients.10,11 Alternate-day corticosteroid regimens are not recommended because they have been associated with rebound arteritis.19
Prognosis. Recent research suggests that recovery of vision is unlikely even when intravenous corticosteroids are administered immediately.20 Stabilization of vision during the first week of treatment appears to be the key factor in preventing further vision loss.16 ■
References:
1. Nordborg E, Bengtsson BA. Epidemiology of biopsy-proven giant cell arteritis (GCA). J Intern Med.1990;227:233-236.
2. Hayreh SS, Podhajsky PA, Raman R, Zimmerman B. Giant cell arteritis: validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285-296.
3. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125:509-520.
4. Hayreh SS, Podhajsky PA, Zimmerman B. Occult giant cell arteritis: ocular manifestations. Am J Ophthalmol. 1998;125:521-526.
5. Hayreh SS, Zimmerman B. Management of giant cell arteritis: our 27-year clinical study: new light onold controversies. Ophthalmologica. 2003;217:
239-259.
6. Liu GT, Glazer JS, Schatz NJ, Smith JL. Visual morbidity in giant cell arteritis: clinical characteristicsand prognosis for vision. Ophthalmology. 1994;101:1779-1785.
7. Goodwin JA. Temporal arteritis: diagnosis and management. In: Focal Points: Clinical Modules for Ophthalmologists. vol. 10, no. 2. San Francisco, CA:American Academy of Ophthalmology; 1992:1-11.
8. Lee AG, Brazis PW. Giant cell arteritis. In: Focal Points: Clinical Modules for Ophthalmologists. vol. 23, no. 6. San Francisco, CA: American Academy of Ophthalmology; 2005:1-13.
9. Skorin L. Neuro-ophthalmic disorders. In: Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology. 4th ed. Boston, MA: Butterworth Heinemann; 2001:457-459.
10. Kanski J. Neuro-ophthalmology. In: Kanski J, ed. Clinical Ophthalmology. 4th ed. Boston, MA: Butterworth Heinemann; 2000:593-597.
11. Goodwin J. Temporal arteritis, neuro-ophthalmic disease. In: Onofrey BE, Skorin L, Holdeman NR, eds. Ocular Therapeutics Handbook: A Clinical Manual. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:603-606.
12. Galetta S. Vasculitis. In: Miller NR, Newman NJ, eds. Walsh & Hoyt’s Clinical Neuro-Ophthalmology. 6th ed. vol. 2. Philadelphia, PA: Lippincott Wiliams & Wilkins; 2005:2333-2426.
13. Foroozan R, Danesh-Meyer H, Savino PJ, et al. Thrombocytosis in patients with biopsy-proven giantcell arteritis. Ophthalmology. 2002;109:1267-1271.
14. Pless M, Rizzo JF 3rd, Lamkin JC, Lessell S. Concordance of bilateral temporal artery biopsy ingiant cell arteritis. J Neuroophthalmol. 2000;20:
216-218.
15. Farris B. Temporal artery biopsy. In: Focal Points: Clinical Modules for Ophthalmologists. vol. 21, no. 7. San Francisco, CA: American Academy of Ophthalmology; 2003:1-10.
16. Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis. Ophthalmology. 2005;112:744-756.
17. Beri M, Klugman MR, Kohler JA, Hayreh SS. Anterior ischemic optic neuropathy, VII: incidence of bilaterality and various influencing factors. Ophthalmology. 1987;94:1020-1028.
18. Miller NR, Keltner JL, Gittinger JW. Giant cell (temporal) arteritis. The differential diagnosis. Surv Ophthalmol. 1979;23:259-263.
19. Hunder GG, Sheps SG, Allen GL, Joyce JW. Daily and alternate-day corticosteroid regimens in treatment of giant cell arteritis: comparison in a prospective study. Ann Intern Med. 975;82:613-618.
20. Danesh-Meyer H, Savino PJ, Gamble G. Poor prognosis of visual outcome after visual loss fromgiant-cell arteritis. Ophthalmology. 2005;112:1098-1103.


