Thrombolytics in Acute Pulmonary Embolism Complicated by Refractory Hypoxemia
 The morbidity and mortality of acute pulmonary embolism remain high despite advances in imaging techniques for rapid and accurate diagnosis.1-3 Treatment options are limited and directed toward anticoagulation to prevent progression of an already-formed thrombus.
The morbidity and mortality of acute pulmonary embolism remain high despite advances in imaging techniques for rapid and accurate diagnosis.1-3 Treatment options are limited and directed toward anticoagulation to prevent progression of an already-formed thrombus.
Based on randomized controlled trials, the use of thrombolytics has been limited to a small patient population with cardiogenic shock and/or cardiopulmonary arrest.4 The purpose is to provide rapid resolution of pulmonary artery thrombus and return of normal hemodynamic parameters. The following case report describes the use of thrombolytics in a patient without hemodynamic instability but with persistent hypoxemia from massive bilateral pulmonary embolism.
HISTORY
A 62-year-old man presented to the emergency department with the chief complaint of acute dyspnea. He denied fevers, chills, productive cough, or chest pain and had been in his usual state of health before this episode. His medical and surgical history was significant for hypertension and a recent ankle fracture that required fusion surgery and immobilization. He quit smoking in the 1960s and recently underwent an exercise treadmill test, which demonstrated no evidence of myocardial ischemia.
PHYSICAL EXAMINATION
He was in acute respiratory distress with perioral cyanosis and a respiration rate of 38 breaths per minute. There was also marked use of accessory muscles of respiration. Temperature was 35.8°C (96.4°F); heart rate, 116 beats per minute (sinus); and blood pressure, 154/98 mm Hg. Oxygen saturation by digital pulse oximetry was 82% while receiving 100% oxygen supplementation via a non-rebreather mask. His lungs were clear to auscultation, and cardiovascular examination revealed no jugular venous distension and normal heart tones without murmur, gallop, or rub.
LABORATORY AND RADIOGRAPHIC FINDINGS
White blood cell count was 14,600/µL with normal hemoglobin and platelet values. The serum bicarbonate level was 17 mEq/L, and the rest of the electrolyte and liver function panels were within normal limits. His B-type natriuretic peptide level was 31 pg/mL. CK-MB and troponin-I levels were 8.75 ng/mL and 1.26 ng/mL, respectively. D-dimer level was 3286 µg/L. Arterial blood gas measurement while receiving 100% FiO2 supplementation via a non-rebreather mask revealed a pH of 7.39; PCO2, 35 mm Hg; PO2, 46 mm Hg; and HCO3-,20 mmol/L. Chest radiography showed clear lungs with unremarkable pulmonary vasculature.
 HOSPITAL COURSE
HOSPITAL COURSE
The patient’s hypoxic respiratory failure required endotracheal intubation and mechanical ventilation. Oxygen saturation remained inadequate despite maximal FiO2 supplementation. Because pulmonary embolism was strongly suspected, 90 mg of subcutaneous enoxaparin was administered empirically.
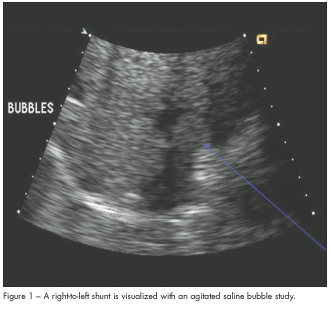
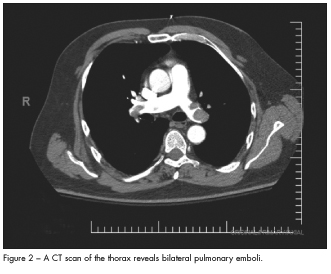
A bedside echocardiogram demonstrated a severely dilated right ventricle with moderately reduced right ventricular (RV) systolic function, mild to moderate tricuspid regurgitation, normal left ventricular (LV) size and function, and an estimated pulmonary pressure of 40 mm Hg. A large right-to-left interatrial shunt was revealed using an agitated saline bubble study (Figure 1). Bilateral pulmonary emboli involving the right mid and distal main pulmonary artery and the proximal and distal left main pulmonary artery were evident on a spiral CT scan (Figure 2).
The patient was transferred to the intensive care unit, and thrombolytic therapy was initiated with alteplase, 100 mg infusion over 2 hours followed by administration of unfractionated heparin. Shortly after, oxygen saturation normalized. The patient tolerated the treatment without complications and was extubated 3 days later. A Doppler study of the lower extremities confirmed the presence of acute deep vein thrombosis throughout the mid-distal superficial femoral, popliteal, and posterior tibial veins of his left lower extremity. An inferior vena cava filter was placed, and warfarin was initiated prior to the patient’s discharge from the hospital.
A follow-up 2-dimensional echocardiogram 2 months later demonstrated normalization of RV function. There was a persistent patent foramen ovale, although the degree of right-to-left shunting had decreased significantly (Figure 3).
DISCUSSION
The standard treatment for acute pulmonary embolism is anticoagulation with either unfractionated heparin or low-molecular-weight heparin, followed by warfarin. Unfractionated heparin or low-molecular-weight heparin is discontinued after adequate overlap of the target INR is achieved.
While there are not a large amount of data supporting the use of thrombolytics in this setting, a meta-analysis demonstrated some benefit with thrombolytic therapy in a subset of high-risk patients presenting with acute pulmonary embolism.4 Guidelines set forth by the European Society of Cardiology and the American College of Chest Physicians recommend the use of thrombolytic therapy in patients with hemodynamic compromise consisting of refractory hypotension or shock.5
Another indication for thrombolytic therapy is in patients who are normotensive but have evidence of RV dysfunction. Echocardiographic evidence of RV dysfunction in patients with acute pulmonary embolism has been shown to be a marker of significantly increased mortality when compared to patients without evidence of RV dysfunction.6 Consequently, it has been proposed that hemodynamically stable patients with acute pulmonary embolism should undergo risk stratification with echocardiography, and those with moderate or severe RV dysfunction should receive thrombolytics.

In massive acute pulmonary embolism, the presence of severe RV dysfunction leads to decreased LV preload and subsequently decreased cardiac output. This patient’s echocardiogram demonstrated moderate RV dysfunction and the presence of a large patent foramen ovale with right-to-left shunting by agitated saline bubble study. The presence of this large right-to-left shunt likely explains his profound and persistent hypoxemia, as well as the lack of hemodynamic instability. This patient’s interatrial septal defect allowed for adequate LV filling by allowing decompression of the right ventricle and shunting of blood into the left ventricle.
The presence of profound and persistent hypoxemia stimulated the use of more aggressive therapy with thrombolytics despite normal hemodynamics. Extensive search of the literature revealed only two cases of persistent hypoxemia secondary to the presence of an interatrial septal defect in the setting of acute pulmonary embolism.7,8
CONCLUSION
There are few guidelines regarding the use of thrombolytic therapy in the acute management of pulmonary embolism. Current recommendations are to consider thrombolytic therapy for acute pulmonary embolism in the subset of patients who present with hemodynamic instability, or those who are normotensive but have evidence of severe RV dysfunction.
The presence of refractory hypoxemia in normotensive patients with acute pulmonary embolism may be yet another indication for thrombolytic therapy. The physiologic derangement in these patients may be as severe as in those who demonstrate hypotension and shock. The presence of an interatrial shunt may mask such physiologic disturbance by allowing for adequate LV filling pressures and cardiac output. While we do not advocate evaluating every patient with acute pulmonary embolism for right-to-left shunting, the presence of severe and persistent hypoxemia despite standard anticoagulation therapy may be a clue to the presence of this defect and the need for thrombolytic therapy.
1. Goldhaber SZ, Elliott CG. Acute pulmonary embolism: part II: risk stratification, treatment, and prevention. Circulation. 2003;108:2834-2838.
2. Goldhaber SZ, Visani L, De Rosa M, et al. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER).Lancet. 1999;353:1386-1389.
3. Goldhaber SZ. Pulmonary embolism. Lancet. 2004;363:1295-1305.
4. Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110:744-749.
5. European Society of Cardiology. Task Force on Pulmonary Embolism. Guidelines on diagnosis and management of acute pulmonary embolism. Eur Heart J.2000;21:1301-1336.
6. Konstantinides S, Geibel A, Heusel G, et al. Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
7. Oostenburg M. Three patients with massive pulmonary embolism. Ned Tijdschr Geneeskd. 2001;145:1277-1281.
8. Keeble T, Lewis J, Ho KM. Massive pulmonary embolism without hemodynamic compromise caused by presence of a patent foramen ovale. Heart. 2006;92:109.
FOR MORE INFORMATION:
- Arcasoy SM. Thrombolytic therapy of pulmonary embolism. A comprehensive review of current evidence. Chest. 1999;115:1695-1707.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341:507-511.
- Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry.J Am Coll Cardiol. 1997;30:1165-1171.
- Konstantinides S, Geibel A, Olschewski M, et al. Association between thrombolytic treatment and the prognosis of hemodynamically stable patients with major pulmonary embolism: results of a multicenter registry. Circulation. 1997;96:882-888.
- McRae SJ, Ginsberg JS. Initial treatment of venous thromboembolism. Circulation. 2004;110[suppl I]:I-3-I-9.
- Stein P, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.


