Peripheral Arterial Disease: Diagnosis and Treatment
ABSTRACT: The classic symptom of peripheral arterial disease (PAD) is intermittent claudication (IC); however, the ankle-brachial index, which is the Doppler ultrasound–recorded ratio of the systolic blood pressure in the ankle to that in the arm, is a much more sensitive method of diagnosis than subjective reports of IC. Among the major risk factors for PAD are advanced age, smoking, diabetes mellitus, hyperlipidemia, and hypertension. Based on its association with cardiovascular risk, PAD is now recognized as a coronary disease equivalent. Patients with PAD should therefore be managed with aggressive risk factor modification. A supervised aerobic exercise program based on walking or other forms of exercise involving the repetitive use of the major leg muscles should be recommended as an initial treatment for patients with IC. Revascularization should be considered in patients with rest pain, tissue loss, or significant lifestyle limitations.
______________________________________________________________________________________________________________________________
Peripheral arterial disease (PAD), or arteriosclerosis obliterans, refers to occlusive atherosclerotic disease of the aorta and of the renal, mesenteric, and lower extremity arteries.1 The classic symptom of PAD is intermittent claudication, defined as pain, aching, and fatigue or discomfort in the lower extremities that is reproducible by exercise and relieved by rest. Claudication can lead to a significant decline in affected patients’ peak exercise performance, to as much as 50% that of age-matched controls, an impairment equivalent to that of moderate to severe heart failure, according to New York Heart Association criteria. Identifying PAD is important because of its association with increased cardiovascular and overall mortality, functional impairment, and functional decline.

EPIDEMIOLOGY AND PREVALENCE
The reported prevalence of PAD varies and is based on the diagnostic methods used for evaluation. In 2 studies that addressed the prevalence of symptomatic and asymptomatic PAD in an elderly population and the general population, respectively, the prevalence of patient-reported symptomatic intermittent claudication ranged from approximately 1% to 5%, with a higher rate observed in older patient populations.2,3 However, in patients with PAD diagnosed according to the ankle-brachial index (ABI), which is the Doppler ultrasound–recorded ratio of the systolic blood pressure in the ankle to that in the arm, the prevalence of intermittent claudication varies from approximately 10% to 30% (Table 1).4 Therefore, patient-reported symptoms of intermittent claudication, a much less reliable method of diagnosing PAD, may lead to an underestimation of the prevalence of this disease. Most epidemiologic studies have used the ABI to diagnose PAD. In these large population-based studies, the prevalence of PAD based on abnormal ABI ranged from 4.6% to 29.0%.5
Among patients with PAD, severe disease, indicated by an ABI of less than 0.5, is associated with increased mortality.6 The Strong Heart Study7 demonstrated a significant association between elevated ABI values (ie, higher than 1.40) and mortality. Elevated ABI values (ie, higher than 1.30 or higher than 1.40) indicate the presence of noncompressible lower extremity arteries that preclude accurate measurement of ankle systolic pressure. However, noncompressible arteries may indicate the presence of medial artery calcification. Persons with ABI values greater than 1.40 have a higher prevalence of classic intermittent claudication symptoms and atypical exertional leg symptoms relative to persons with normal ABIs (0.90 to 1.30), suggesting the possibility of an increased prevalence of PAD among those with elevated ABI values. The magnitude of both total and cardiovascular mortality risk with an ABI of greater than 1.40 was similar to that with an ABI of less than 0.90.7
Death that results primarily from the complications of PAD is rare. Mortality is caused mainly by concomitant coronary and cerebrovascular disease. The relative risk of death from all causes is 3.1 in patients with PAD compared with the general population.8 The relative risk of death from coronary heart disease is 6.9, accounting for more than two thirds of the mortality in this study population.9 The associations between PAD and cardiovascular mortality are independent of age, body mass index, cigarette smoking, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, blood pressure, fasting glucose level, and history of angina, myocardial infarction, stroke, or other heart problems.7
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Atherosclerosis accounts for the vast majority of cases of PAD. Atherosclerotic plaques frequently develop at branch points in arteries mainly in response to the effects of disturbed blood flow on endothelial cells.10 These plaques contain inflammatory cells (monocytes, macrophages, and lymphocytes) in addition to a thrombogenic lipid core that is covered by a fibrous cap.11
The “response-to-injury” theory is most widely accepted. Endothelial injury due to hemodynamic stress causes vascular inflammation and triggers a fibroproliferative response. Once the fibrous cap is disrupted, the resultant exposure of the prothrombogenic lipid core can lead to thrombus formation and flow occlusion.10,11 Other factors associated with this occlusive mechanism are impaired vasodilatation due to decreased endothelium-derived nitric oxide, and aggravated vasoconstriction due to increase in inflammatory factors, mainly thromboxane, serotonin, angiotensin II, endothelin, and norepinephrine.10,11
Patients with severe limb ischemia have poorly perfused skin capillaries, mainly owing to reduced red blood cell deformability, increased leukocyte adhesiveness, platelet aggregation, and excessive vasoconstriction. These abnormalities in the microcirculation contribute to the pathophysiology of critical limb ischemia.12
RISK FACTORS
The risk factors for PAD are the same as those for coronary artery disease. Among these factors, diabetes mellitus and smoking increase the risk independently by approximately 2- to 4-fold.13 The association between smoking and PAD is about twice as strong as that between smoking and coronary artery disease.14 Diabetes mellitus is a stronger risk factor for PAD and accounts for 50% to 70% of all nontraumatic amputations in the United States. Since diabetic neuropathy may often mask PAD symptoms, PAD is more commonly asymptomatic in patients with diabetes. In addition, PAD is more severe and rapidly progressive in patients with diabetes than in those without diabetes, and it presents later in life.13,15 Diabetes is a stronger risk factor for PAD in women than in men.15
Advanced age is associated with an increased prevalence of PAD (0.9% among persons aged 40 to 49 years and 14.5% among those 70 years and older).13 The prevalence increases by 1.5- to 2.0-fold for every 10-year increase in age. Patients with chronic kidney disease and hyperlipidemia are at increased risk for PAD. African American and Hispanic patients have a higher tendency to develop PAD independent of other risk factors (Table 2).
Other risk factors include hypertriglyceridemia; elevated levels of the inflammatory markers C-reactive protein, fibrinogen, and interleukin-6; homocysteinemia; elevated levels of lipoprotein(a), antiphospholipid antibodies, and D-dimer; hypercoagulable states; and an abnormal waist-to-hip ratio.
CLINICAL FEATURES
Claudication, ischemic rest pain, and skin ulceration are the usual presenting symptoms of PAD. The location of the symptom often relates to the site of the most proximal stenosis. Thus, a patient with pain in the hip, buttock, or thigh is likely to have an aortoiliac stenosis (Table 3). Calf pain is typically due to femoropopliteal disease, and foot claudication is the result of tibial disease. Claudication from ischemic sources occurs in muscle groups—not in joints. Relief with rest is therefore independent of position and complete, usually occurring within 5 minutes.
Weak or absent pulses are the hallmark physical finding of PAD. A decreased or absent pulse is important in diagnosing the location of stenosis that is proximal to the palpated artery (Table 4). Bruits represent accelerated blood flow velocity and flow disturbance at sites of stenosis. Other physical findings include thinning of the skin, a smooth and shiny appearance of the skin, subcutaneous fat atrophy, loss of hair on the limb, thickening of the nails, pallor induced by elevation of the limb above the heart, muscle atrophy, and ulceration over the affected segments. Arterial ulcers vary in size, measuring usually between 3 and 5 cm in diameter, have a pale base, and are well-circumscribed lesions typically appearing after trauma or injury to a limb and involving the tips of the toes or the heel.
The differential diagnosis of intermittent claudication is given in Table 5. Resting pain (claudication) in a limb can indicate critical limb ischemia, which can often result in nonhealing ulcers or gangrene that leads to limb loss in the absence of treatment. In one study, 12.2% of patients with critical limb ischemia progressed to major amputation within 3 months and 20% died within 1 year.13,16 Another study has shown that the cumulative 10-year rates of progression from intermittent claudication to ischemic ulceration and rest pain were 23% and 30%, respectively.17
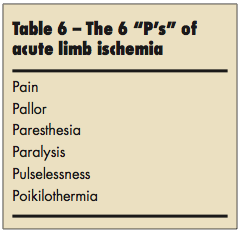
Acute limb ischemia is a vascular emergency due to an acute occlusive event more typically caused by thrombus rather than by embolus. This event may lead to irreversible ischemia, permanent neuromuscular damage, or major amputation regardless of therapy. The clinical features of acute limb ischemia include the 6 “P’s”: pain, pallor, paresthesia, paralysis, pulselessness, and poikilothermia (Table 6). Of these, paresthesias and paralysis usually represent irreversible ischemic injury.

Continued on next page
DIAGNOSIS
According to the American College of Cardiology (ACC) guidelines,1 noninvasive screening (usually an ABI evaluation) should be performed for patients with suspected lower extremity PAD, who are defined as persons at least 70 years old with exertional leg symptoms and nonhealing wounds or at least 50 years old with these symptoms and a history of smoking or diabetes (Table 7). The American Diabetes Association suggests that a screening ABI evaluation be performed for patients with diabetes who are younger than 50 years and have risk factors for PAD.18
 The ABI evaluation is a noninvasive, inexpensive, simple, and effective diagnostic test that can be performed easily in an ambulatory setting. To provide accurate assessment, the systolic blood pressure is recorded in both brachial arteries and in the dorsalis pedis and posterior tibial arteries. The ABI is calculated for each leg by dividing the highest ankle systolic pressure by the highest brachial systolic pressure, and the value is recorded to 2 decimal places. In healthy persons, owing to the higher peripheral resistance at the ankles, the ankle pressure will be 10 to 15 mm Hg more than the brachial pressure. An ABI of less than 0.90 is considered abnormal and is 95% sensitive for angiographically confirmed peripheral arterial stenosis (see Table 1).
The ABI evaluation is a noninvasive, inexpensive, simple, and effective diagnostic test that can be performed easily in an ambulatory setting. To provide accurate assessment, the systolic blood pressure is recorded in both brachial arteries and in the dorsalis pedis and posterior tibial arteries. The ABI is calculated for each leg by dividing the highest ankle systolic pressure by the highest brachial systolic pressure, and the value is recorded to 2 decimal places. In healthy persons, owing to the higher peripheral resistance at the ankles, the ankle pressure will be 10 to 15 mm Hg more than the brachial pressure. An ABI of less than 0.90 is considered abnormal and is 95% sensitive for angiographically confirmed peripheral arterial stenosis (see Table 1).
In patients with calcified or noncompressible arteries, an ABI may underestimate the severity of PAD. In such patients, the toe brachial index should be determined, because small arteries are less susceptible to calcification. A toe brachial index of less than 0.70 confirms the diagnosis of PAD.
A pulse volume recording study is a visual examination of the plethysmographic tracings that detect changes in the volume of blood flow through the limb. Because of the qualitative nature of this test, the results may be less accurate than those from duplex ultrasonography for localizing a lesion. Pulse volume recording is often used to follow the status of lower extremity revascularization procedures.
An exercise ABI should be determined for a patient with high clinical suspicion for PAD who has a normal resting ABI.1 It measures the functional limitation of claudication as well as the patient’s response to therapy. It should not be performed in patients with ischemic rest pain.
Other tests include arterial duplex ultrasonography, magnetic resonance angiography (MRA), computed tomography angiography (CTA), and standard angiography. Duplex ultrasonography is useful to determine the anatomic location and severity of stenosis in PAD. This screening technique measures flow velocities and identifies obstructive lesions. It is recommended for routine surveillance after femoropopliteal or femorotibial/pedal bypass surgery with a venous conduit. The minimum surveillance intervals are approximately 3, 6, and 12 months and then yearly after graft placement (American Heart Association [AHA] class IA indication).1

MRA is more sensitive and specific than duplex ultrasonography in determining the location and severity of stenosis and may be useful in determining the possible surgical and endovascular revascularization options in patients with severe disease.19
The use of CTA as a diagnostic method in PAD has increased with the advent of 64-channel “multidetector” scanners. In contrast to MRA, CTA has higher resolution and can provide images of calcification in the vessel wall. Patients with pacemakers or defibrillators can undergo CTA safely. The ACC/AHA practice guidelines on the diagnosis and treatment of PAD suggest that CTA may be useful in planning revascularization strategies and in determining the anatomic location and severity of stenosis in patients with lower extremity PAD (class IIb) and in those who have contraindications to MRA (see Table 7).1
Although angiography remains the “gold standard” for evaluating the anatomy of the peripheral arterial system, it is invasive and carries certain risks such as contrast nephropathy, bleeding, and emboli. Therefore, it should be used only when the diagnosis of PAD is in question or an endovascular or surgical procedure is planned.
Continued on next page
TREATMENT
Aggressive risk factor modification is the cornerstone of therapy for all patients with PAD, for whom the goal is to prevent myocardial infarction, stroke, and death (Table 8). Cessation of smoking is the most cost-effective measure available. The rate of progression and amputation in patients with PAD who continue to smoke is more than 2-fold higher than in those who quit.20 All patients with PAD who smoke should be offered comprehensive smoking cessation interventions, including behavior modification therapy, nicotine replacement therapy, and pharmacologic therapy such as bupropion and varenicline.

Aspirin administered in daily doses of 75 to 325 mg is recommended as safe and effective antiplatelet therapy for reducing the risk of myocardial infarction, stroke, and vascular death in persons with atherosclerotic lower extremity PAD. Clopidogrel (75 mg/d) is recommended as an effective alternative antiplatelet therapy to aspirin.
Oral anticoagulation therapy with warfarin has not been shown to reduce the risk of cardiovascular ischemic events in persons with atherosclerotic lower extremity PAD and is therefore not recommended.1 All patients with PAD should receive statin therapy unless it is contraindicated.1 A low-density lipoprotein cholesterol target of less than 100 mg/dL for patients with PAD and a target of less than 70 mg/dL for patients with significant cardiovascular risk factors are recommended.1 Statins may increase patients’ exercise tolerance and improve their pain-free walking ability.21
For patients with lower extremity PAD who have hypertension, antihypertensive therapy should be administered to achieve a goal of less than 140/90 mm Hg in patients without diabetes or less than 130/80 mm Hg in patients with diabetes and those with chronic kidney disease.1 Beta-adrenergic blocking drugs are effective antihypertensive agents and are not contraindicated in most patients with PAD. Angiotensin-converting enzyme inhibitors can be used by symptomatic patients with lower extremity PAD to reduce the risk of adverse cardiovascular events. In a comparison with placebo in patients with PAD, angiotensin-converting enzyme inhibitor therapy also led to significant increases in mean pain-free walking time and maximum walking time.22
For patients with PAD who have diabetes, the American Diabetes Association guidelines23 recommend aggressive treatment with oral medications, insulin, or both to achieve a hemoglobin A1c goal of less than 7.0%. Proper foot care, including daily foot inspection, skin cleansing, and use of topical moisturizing creams, should be encouraged and skin lesions and ulcerations should be addressed urgently in all patients with diabetes and lower extremity PAD.23 The effectiveness of folic acid and B12 vitamin supplements in persons with lower extremity PAD and homocysteine levels greater than 14 mmol/L is not well established.
A dedicated and supervised aerobic exercise program based on walking or other forms of exercise involving the repetitive use of the major leg muscles is important and should be recommended as an initial treatment for patients with intermittent claudication.1 The supervised exercise training should be performed for a minimum of 30 to 45 minutes in sessions performed at least 3 times weekly for a minimum of 12 weeks to improve walking distance.24
FDA-approved pharmacologic treatment. Two pharmacologic agents are approved by the US Food and Drug Administration for the treatment of intermittent claudication: cilostazol and pentoxifylline. The ACC and AHA recommend cilostazol as an effective first-line therapy for reducing symptoms and increasing walking distance among patients with PAD and intermittent claudication in the absence of heart failure.1 Cilostazol, a phosphodiesterase type 3 inhibitor that increases cyclic adenosine monophosphate, has vasodilator and antiplatelet effects.25 The recommended dose of cilostazol is 100 mg orally twice daily. Common adverse effects include gastrointestinal symptoms (nausea, diarrhea, and abnormal stool), headache, and palpitations. In comparisons with placebo, other phosphodiesterase inhibitors have been associated with decreased survival in patients with class III to IV congestive heart failure.26 Cilostazol is contraindicated in patients with congestive heart failure of any severity.26
Pentoxifylline, a methylxanthine derivative, is a hemorheologic agent. It decreases blood viscosity, increases red blood cell and leukocyte deformability, and reduces platelet adhesion.25 The recommended dose is 400 mg orally 3 times daily. Gastrointestinal symptoms are the most common adverse effects. Cilostazol and pentoxifylline appear to improve functioning and increase walking distance slowly over time. At least 12 weeks of therapy is recommended before discontinuing the medication if an adequate response is not evident.
Investigational treatments. Other treatments that are promising but still under investigation are L-carnitine and propionyl-L-carnitine, recombinant fibroblast growth factor 2, L-arginine, prostaglandin derivatives, naftidrofuryl and buflomedil, and immunomodulation therapy. Their efficacy and potential role remain to be better defined.
Endovascular and surgical treatment. Revascularization should be considered in the patient with rest pain, tissue loss, or significant lifestyle limitations. Surgical revascularization has been available for years. Large-vessel bypass surgery with vein or synthetic graft material is well established.27 Recent advances in interventional therapy employing percutaneous transluminal angioplasty (PTA), with or without stent placement, may be relaxing the indications for endovascular treatment previously reserved only for severe PAD (Figure).
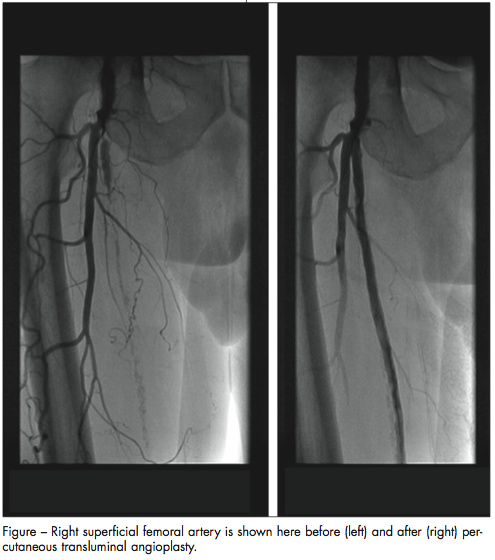
Endovascular techniques for treating peripheral arterial occlusive disease, including PTA with balloon dilation, stents, atherectomy, laser surgery, cutting balloons, thermal angioplasty, and fibrinolysis/fibrinectomy, are increasingly used in different subgroups of patients. PTA is best attempted—and provides optimal results—in patients with a focal segmental stenosis in a large vessel. The technique is the most commonly used percutaneous strategy for the treatment of infra-inguinal occlusive disease, whereas surgery is best applied to multilevel stenosis in smaller and more distant vessels.
The iliac arteries are most amenable to endovascular therapy. Studies have shown an 88% success rate for recanalization of occluded common or external iliac arteries28 and a 5-year cumulative patency rate as high as 66%.29 Currently, there is an ACC/AHA class IB recommendation for percutaneous intervention for a single stenosis of less than 3 cm within the common iliac, external iliac (unilateral/bilateral), superficial femoral, or popliteal artery.1 The use of drug-eluting stents is still investigational. Patients who have long occlusions (more than 5 cm), iliac artery aneurysmal disease, atheroembolic disease, and long-segment, severe diffuse bilateral aorto-iliac disease are not optimal candidates for endovascular percutaneous therapy.
Endovascular therapy for femoropopliteal disease has historically been associated with high rates of restenosis, ranging from 50% to 80%.30 There have been advances in stent design and atherectomy devices that have improved the 1-year patency rates, making this a good option for most patients. The ACC/AHA guidelines on the treatment of PAD recommend against primary stent placement in the femoral, popliteal, or tibial arteries, but do give a class IIa recommendation to stent placement for a failed balloon angioplasty (more than 50% residual stenosis after balloon angioplasty).1 Surgical bypass grafting is the treatment of choice in the initial treatment for critical limb ischemia when severe multilevel disease is present. Despite the advantages of PTA over surgical revascularization in certain cases, it is important to point out the decreasing long-term effectiveness of PTA in distal vessels and long stenoses. However, for patients at high risk for limb loss (who are deemed poor surgical candidates or technically unsuitable for surgical revascularization), it is reasonable to consider distal angioplasty for limb salvage.
CONCLUSIONS
Based on its association with cardiovascular risk, PAD is now recognized as a coronary disease equivalent. Patients with PAD should therefore be managed with aggressive secondary prevention. Mechanical intervention should be employed when acute limb ischemia, critical limb ischemia, or lifestyle-limiting intermittent claudication is evident. Further advances are expected in endovascular techniques for diagnosing, treating, and preventing PAD.
Acknowledgments: The authors would like to thank Caroline Murray for her efforts and dedication in the preparation of this manuscript.
1. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2006 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). J Vasc Interv Radiol. 2006;17:1383-1398.
2. Meijer WT, Hoes A, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185-192.
3. Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1991;20:384-392.
4. McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA.2001;286:1599-1606.
5. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738-743.
6. McDermott MM, Feinglass J, Slavensky R, Pearce WH. The ankle-brachial index as a predictor of survival in patients with peripheral vascular disease. J Gen Intern Med.1994;9:445-449.
7. Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality. The Strong Heart Study. Circulation. 2004;109:733-739.
8. Criqui M, Langer R, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
9. Lassila R, Lepantalo M, Lindfors O. Peripheral arterial disease—Natural outcome. Acta Med Scand. 1986;220:295-301.
10. Faxon DP, Fuster V, Libby P, et al. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation. 2004;109:2617-2625.
11. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115-126.
12. Bollinger A, Hoffmann U, Franzeck UK. Microvascular changes in arterial occlusive disease: target for pharmacotherapy. Vasc Med. 1996;1:50.
13. Murabito JM, D’Agostino RB, Silbershatz H, et al. Intermittent claudication. A risk profile from the Framingham Heart Study. Circulation. 1997;96:44-49.
14. Price JF, Mowbray PI, Lee AJ, et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344-353.
15. Abbott RD, Brand FN, Kannel WB. Epidemiology of some peripheral arterial findings in diabetic men and women: experiences from the Framingham Study. Am J Med. 1990;88:376-381.
16. Aquino R, Johnnides C, Makaroun M, et al. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962-970.
17. Muluk SC, Muluk VS, Kelley ME, et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J Vasc Surg. 2001; 33:251-257; discussion 257-258.
18. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333-3341.
19. Leiner T, Tordoir JH, Kessels AG, et al. Comparison of treatment plans for peripheral arterial disease made with multi-station contrast medium-enhanced magnetic resonance angiography and duplex ultrasound scanning. J Vasc Surg. 2003;37:1255-1262.
20. Jonason T, Bergstrom R. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253-260.
21. Mohler ER III, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation.2003;108:1481-1486.
22. Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Ramipril markedly improves walking ability in patients with peripheral arterial disease. Ann Intern Med. 2006;144:660-664.
23. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333-3341.
24. Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain: a meta-analysis. JAMA. 1995;274:975-980.
25. Jacoby D, Mohler ER III. Drug treatment in intermittent claudication. Drugs. 2004;64:1657-1670.
26. Cilostazol tablets [prescribing information]. Sellersville, PA: TEVA Pharmaceuticals USA; 2012.
27. Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104-114.
28. Colapinto RF, Stronell RD, Johnston WK. Transluminal angioplasty of complete iliac obstructions. AJR Am J Roentgenol. 1986;146:859-862.
29. Schurmann K, Mahnken A, Meyer J, et al. Long-term results 10 years after iliac arterial stent placement. Radiology. 2002;224:731-738.
30. Martin EC. Transcatheter therapies in peripheral and noncoronary vascular disease. Introduction. Circulation. 1991;83(2 suppl):I1-I5.


