Peer Reviewed
Diffuse Large B-Cell Non-Hodgkin Lymphoma Presenting as Acute Pancreatitis
Authors:
Claudia Gordillo, DO
Aventura Hospital, Miami, Florida
Wilson B. Pfeiffer, DO
Brooke Army Medical Center, San Antonio, Texas
Alixandria A. Fiore, DO
University of Texas Health Science Center at San Antonio, Texas
Roberto Comperatore, MD
Palmetto General Hospital, Miami, Florida
Nuria Lawson, MD
Palmetto General Hospital, Miami, Florida
Luis C. Rey, MD
Palmetto General Hospital, Miami, Florida
Syed A. A. Rizvi, PhD, MS, MBA
Hampton University School of Pharmacy, Hampton, Virginia
Mileydis Alonso, DO
Cleveland Clinic Florida, Weston, Florida
Andres Rodriguez, DO
University of Miami–Jackson Memorial Hospital, Miami, Florida
Citation:
Gordillo C, Pfeiffer WB, Fiore AA, Comperatore R, Lawson N, Rey LC, Rizvi SAA, Alonso M, Rodriquez A. Diffuse large B-cell non-Hodgkin lymphoma presenting as acute pancreatitis. Consultant. 2019;59(10):313-315.
A 70-year-old man with a past medical history of hyperlipidemia, benign prostatic hyperplasia, and cholecystectomy presented to the emergency department (ED) with abdominal pain, vomiting and abdominal distention for the past 9 days.
He stated that the pain was localized to the epigastric region and was constant and bloating in nature. He also reported 3 episodes of vomiting nonbloody, dark, bilious fluid and reported constipation for several days. He denied any episode of hematochezia; however, he stated that he had experienced straining with bowel movements, resulting in “string-like, pale” stools. The patient denied unexpected weight loss and current or previous use of alcohol, tobacco, of illicit drugs. His family history included a father who died of colon cancer at age 78. He had undergone a colonoscopy 5 years prior, the results of which were negative for gastrointestinal tract pathology.
Physical examination. The patient’s abdomen was tender to both light and deep palpation in all 4 quadrants and was distended and not soft upon palpation. There was no rebound tenderness or voluntary or involuntary guarding of the abdomen, and no palpable masses were found on palpation. The abdomen was dull to percussion in all 4 quadrants. Bowel sounds were present in all 4 quadrants. Anasarca was appreciated, with the most affected areas being the lower extremities and scrotum. The patient was afebrile without leukocytosis and was hemodynamically stable.
Hospital course of stay. The patient was provided intravenous medication for pain upon presentation to the ED. A computed tomography (CT) scan of the abdomen revealed diffusely enlarged and edematous pancreas with surrounding soft tissue stranding and infiltration, along with several adjacent vessels, representing severe pancreatitis (Figure 1).

Figure 1. Axial CT image of interstitial edematous pancreatitis.
Mild intrahepatic biliary dilatation was also noted as a secondary finding, which corresponded with elevated liver function test (LFT) values (alkaline phosphatase, 764 U/L; alanine aminotransferase, 425 U/L; aspartate aminotransferase, 288 U/L). The white blood cell count was elevated at 13.0 million/µL (reference range, 3800-10,800/µL).
The patient was started on intravenous normal saline and made nil per os. Gastroenterology and general surgery consults were obtained, and the patient was admitted to the hospital. Magnetic resonance imaging and ultrasonography of the abdomen showed a markedly enlarged pancreas with severe surrounding inflammatory changes and free fluid. An upper gastrointestinal radiography series revealed no evidence of small bowel obstruction. Lipase and amylase levels continued to be elevated.
To compound the CT finding of intrahepatic biliary dilatation, LFT values and bilirubin levels were persistently elevated. In an effort to find the source of the liver pathology, a hepatitis panel was ordered to screen for hepatitis A, B, and C; results were nonreactive.
Abdominal ultrasonography showed moderate ascites, with the largest pocket of fluid present in the pelvic region. Due to his history of cholecystectomy, nuclear medicine testing of the liver and gallbladder was not pursued. An interventional radiologist was consulted, who proceeded to a percutaneous transhepatic cholangiogram (PTC) with stent placement, which resulted in the draining of 2.8 L of brownish yellow fluid over 24 hours. After the PTC procedure, LFT values and bilirubin levels began to rapidly trend downward until plateauing at mildly elevated levels.
After 10 days of medical management and daily serial CT scans, the pancreatitis had not improved and continued to worsen. CT scans continued to show a progressive enlargement of the pancreatitis; however, there were no signs of necrosis within the pancreas. The patient began having severe dyspnea on day 14; chest radiographs showed an elevation of the right hemidiaphragm due to pleural effusion and increased abdominal pressure. Thoracentesis was performed, draining 1.4 L of serosanguinous fluid, but this provided only minimal relief to the patient. Due to worsening abdominal distension, paracentesis was performed as an attempt to relieve the pressure. Ascitic and pleural fluid were sent for pathological analysis, and bladder pressures were obtained to monitor for abdominal compartment syndrome. Bladder pressures remained at 13 cm H2O, ruling out compartment syndrome.
The ascitic fluid pathology report showed malignant cells consistent with high-grade non-Hodgkin lymphoma (NHL) (Figures 2 and 3).


Figures 2 and 3. Ascitic fluid pathology results were consistent with high-grade NHL.
At this point, a hematologist/oncologist was consulted, and a bone marrow biopsy was performed with the purpose of determining the extent and staging of the lymphoma. The bone marrow biopsy pathology report showed extensive involvement by non-Hodgkin high-grade B-cell lymphoma and a monotypic B-cell population with CD-10 coexpression (Figure 4).
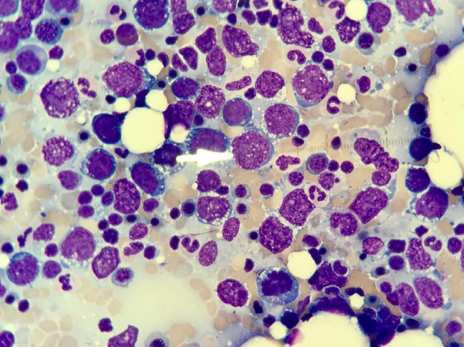
Figure 4. Bone marrow biopsy results showed extensive involvement by non-Hodgkin high-grade B-cell lymphoma and a monotypic B-cell population with CD-10 coexpression.
The patient was started on a cycle of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone and was discharged with continued chemotherapy and outpatient monitoring planned by the hematologist/oncologist.
Discussion. Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy, comprising 31% of all NHLs in Western countries and accounting for 4% of all reported cancer cases in the United States.1-3 The lymphoma can present as enlarged lymph nodes at the primary site or as enlargement of extranodal tissues. At times, DLBCL can arise in extranodal sites such as the liver, large intestine, and small intestine, among others.1 While the lymphoma can arise in virtually any tissue in the body, the most common site of extranodal involvement is the stomach, comprising 68% to 75% of all cases, with the small bowel following at 9%, the ileocecal region at 7%, the rectum at 2%, and diffuse colonic involvement at 1%.4,5
DLBCL is very heterogeneous in gene expression, and so far more than 150 genetic driver genes of this disease have been identified, but the exact causes are still unknown.6,7 The clinical presentation of DLBCL ranges from generalized B symptoms to specific somatic complaints.8
In very rare cases, DLBCL can present with pancreatic involvement, accounting for 0.2% to 2% of all cases.1 To our knowledge, there has been only one other published case of secondary pancreatic involvement of a diffuse large B-cell NHL presenting as acute pancreatitis.9 Our patient presented with clinical signs of acute pancreatitis, which was further confirmed with diagnostic testing.
The most common causes of acute pancreatitis include gallstones, alcohol, and hypertriglyceridemia, among others. Our patient adamantly denied ingestion of alcohol and had well-controlled lipid levels. While biliary and liver abnormalities were identified on imaging and laboratory test results, the placement of a biliary stent through transparietal transhepatic cholecystostomy failed to improve the pancreatitis. After exhausting all diagnostic modalities only to have the patient’s status worsen, a last-ditch diagnostic effort was made by sending the ascitic fluid for pathological analysis, which finally shed light on the root problem, a diffuse large B-cell NHL.
This case highlights the importance of ruling out lymphoma in cases of gastrointestinal tract symptoms that fail to improve with conventional treatment modalities.
References:
- Martelli M, Ferreri AJM, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146-17
- Key statistics for non-Hodgkin lymphoma. American Cancer Society website. https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/key-statistics.html. Revised January 8, 2019. Accessed July 31, 2019.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22(5):941-952.
- Koch P, del Valle F, Berdel WE, et al; German Multicenter Study Group. Primary gastrointestinal non-Hodgkin’s lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German multicenter study GIT NHL 01/92. J Clin Oncol. 2001;19(18):3861-3873.
- Papaxoinis G, Papageorgiou S, Rontogianni D, et al. Primary gastrointestinal non-Hodgkin’s lymphoma: a clinicopathologic study of 128 cases in Greece. A Hellenic Cooperative Oncology Group study (HeCOG). Leuk Lymphoma. 2006;47(10):2140-2146.
- Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma. Cell. 2017;171(2):481-494.e15.
- Zhang J, Grubor V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110(4):1398-1403.
- Le Gouill S, Talmant P, Touzeau C, et al. The clinical presentation and prognosis of diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC rearrangement. Haematologica. 2007;92(10):1335-1342.
- Saif MW, Khubchandani S, Walczak M. Secondary pancreatic involvement by a diffuse large B-cell lymphoma presenting as acute pancreatitis. World J Gastroenterol. 2007;13(36):4909-4911.


